Understanding Organic Chemistry

This article provides an overview of organic chemistry, including the unique properties of carbon, the composition of organic compounds, and the concept of isomers.
- Uploaded on | 0 Views
-
 adleywillms
adleywillms
About Understanding Organic Chemistry
PowerPoint presentation about 'Understanding Organic Chemistry'. This presentation describes the topic on This article provides an overview of organic chemistry, including the unique properties of carbon, the composition of organic compounds, and the concept of isomers.. The key topics included in this slideshow are organic chemistry, carbon, compounds, isomers, molecular formula,. Download this presentation absolutely free.
Presentation Transcript
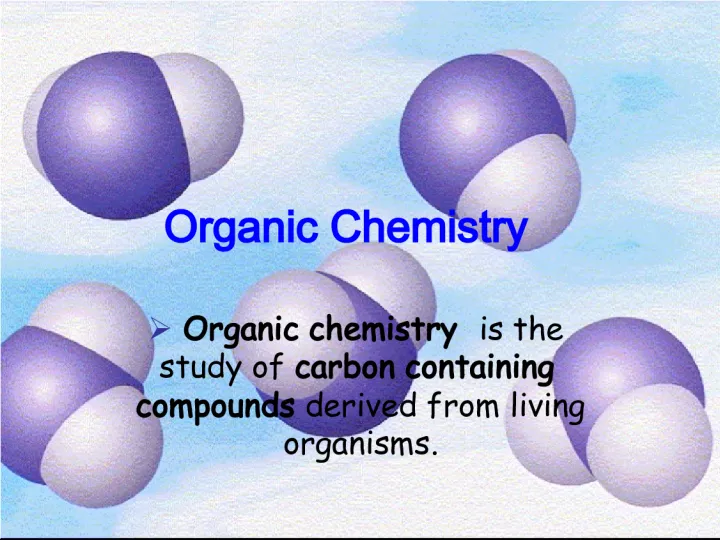
1. Organic Chemistry Organic chemistry is the study of carbon containing compounds derived from living organisms.
2. Organic Compounds Contain C bonded to other elements, commonly H, O, N, S, and halogens Carbon Can form many different compounds due to its hybrid orbitals Has intermediate electonegativity , so its most likely to form molecular compounds ( Recall : molecular compounds have diverse properties) Can make single, double, and triple bonds Can form isomers (same molecular formula but different arrangement of atoms)
3. Isomers Structural Isomers have the same molecular formula but the atoms are bonded together in a different sequence Stereoisomers are molecules with the same molecular formula, a same sequence of atoms but they have different 3D orientations
4. Stereoisomers Diastereomers form around a double bond and each carbon atom involved in the bond must have different types of atoms bonded to it Enantiomers are mirror images of each other
5. Types of Hydrocarbons Saturated: Contain the maximum number of hydrogens, single bonds between all carbons Unsaturated: Contain 1 + double or triple bonds
6. Aliphatic Carbons are arranged in chains Cyclic: Carbons are arranged in rings Aromatic: Contain a benzene ring Types of Hydrocarbons
7. Types of Hydrocarbons
8. Homologous series This is a series of compounds which all contain the same functional group , and have similar chemical properties. ALKANES ALKENES ALCOHOLS CH 4 CH 2 =CH 2 CH 3 OH CH 3 -CH 3 CH 2 =CH CH 3 CH 3 CH 2 OH Each has a general formula : ALKANES: C n H 2n+2 The members of the series differ by the number of CH 2 units. CH 3 -CH 3 , CH 3 - CH 2 -CH 3 , CH 3 - CH 2 -CH 2 -CH 3 Graduation in physical properties : eg: boiling points . CH 4 ( GAS ), C 8 H 18 ( LIQUID ), C 30 H 62 ( SOLID )
9. Rules for Naming Alkanes (Nomenclature) Rules for Naming Alkanes (Nomenclature) For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon 1 2 3 4 4 carbon chain = butane 4 carbon chain = butane
10. Naming Alkanes Based off the number of C atoms in the longest chain 1. Count the number of Cs in the longest chain 2. Determine the appropriate root 3. Add the suffix ane
11. Hydrocarbon Root Names # of Carbons Root Name 1 meth- 2 eth- 3 prop- 4 but- 5 pent- 6 hex- 7 hept- 8 oct- 9 non- 10 dec-
12. Examples Butane Heptane
13. Naming Branched Alkanes Based off the number of C atoms in the longest chain 1. Count the number of Cs in the longest chain 2. Determine the appropriate root 3. Use the numbered Cs to give the branches a position number add yl suffix 4. Add the suffix ane
14. Naming Branched Alkanes Important Rules: 1. Start numbering from the end that will give you the lowest number of branches 2. If there is more than one type of branch, name the branches in alphabetical order 3. If there is more than two of the same type of branch, give the branch a position number and prefixes di, tri tetra etc. 4. Put commas between numbers and hyphens between numbers and letters
15. Rules for Naming Alkanes (Nomenclature) Rules for Naming Alkanes (Nomenclature) When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl . CH 3 Meth yl CH 2 CH 3 Eth yl CH 2 CH 2 CH 3 Prop yl CH 2 CH 2 CH 2 CH 3 But yl Methyl
16. Rules for Naming Alkanes (Nomenclature) Rules for Naming Alkanes (Nomenclature) The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. Methyl 1 2 3 4
17. Rules for Naming Alkanes (Nomenclature) Rules for Naming Alkanes (Nomenclature) The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri- , etc. are used to indicate multiple identical substituents. Methyl 1 2 3 4 Name: 2-methylbutane
18. Nomenclature Practice Nomenclature Practice Name this compound Step #1: For a branched hydrocarbon, the longest continuous chain of carbon atoms gives the root name for the hydrocarbon 1 5 2 4 3 9 6 8 7 9 carbons = nonane
19. Nomenclature Practice Nomenclature Practice Name this compound 1 5 2 4 3 9 6 8 7 9 carbons = nonane Step #2: When alkane groups appear as substituents, they are named by dropping the -ane and adding -yl . CH 3 = methyl chlorine = chloro
20. Nomenclature Practice Nomenclature Practice Name this compound 1 5 2 4 3 9 6 8 7 9 carbons = nonane CH 3 = methyl chlorine = chloro Step #3: The positions of substituent groups are specified by numbering the longest chain of carbon atoms sequentially, starting at the end closest to the branching. 1 9 NOT 9 1
21. Nomenclature Practice Nomenclature Practice Name this compound 1 5 2 4 3 9 6 8 7 9 carbons = nonane CH 3 = methyl chlorine = chloro Step #4: The location and name of each substituent are followed by the root alkane name. The substituents are listed in alphabetical order (irrespective of any prefix), and the prefixes di-, tri- , etc. are used to indicate multiple identical substituents . 2 -chloro- 3 , 6 -dimethylnonane
22. Cyclic Alkanes Cyclic Alkanes Cyclo propane , C 3 H 6 Remember, explicit hydrogens are left out Cyclo butane , C 4 H 8 Cyclo pentane , C 5 H 10 Cyclo hexane , C 6 H 12 Cyclo heptane , C 7 H 14
23. Practice P. 11-16 #1, 2
24. Structural Shorthand Structural Shorthand Explicit hydrogens (those required to complete carbons valence) are usually left off of drawings of hydrocarbons Line intersections represent carbon atoms C 1 C 1 C 2 C 2 C 3 C 3 C 4 C 4
25. Different Methods of Displaying Compounds Molecular Formula Condensed Structural Formula Expanded Molecular Formula Structural Formula Line Formula
26. Naming Alkenes & Alkynes 1. Count the number of Cs in the longest chain containing the double/triple bond. This is the parent chain, determine the root Number the parent chain so that the double/triple bond has the lowest possible position number 2. Identify the position numbers of branches Same rules as before 3. Write the branches in alphabetical order 4. Write the root, including a prefix that identifies the position of the double/triple bond Add the prefix cyclo if its cyclic 5. Add the suffix ene or yne
27. Naming Alkenes & Alkynes
28. Naming Aromatics 1. Same rules 2. If benzene is the parent chain benzene suffix 3. If benzene is a branch group phenyl
29. Practice P. 16-22 #3-7, 8abc Naming Alkenes/Alkynes Worksheet Isomer Challenge Worksheet Naming Hydrocarbons Worksheet