Effector Mechanisms of Humoral Immunity: Antibody Mediated Neutralization, Opsonization, and Complement Activation

This lecture delves into the core effector mechanisms of humoral immunity, including antibody-mediated neutralization, opsonization, complement activation, and IgA-mediated protection. It also discusses how exaggerated complement activation can lead to disease and explains the concept of passive immunity in newborns.
- Uploaded on | 3 Views
-
 albinacyucyura
albinacyucyura
About Effector Mechanisms of Humoral Immunity: Antibody Mediated Neutralization, Opsonization, and Complement Activation
PowerPoint presentation about 'Effector Mechanisms of Humoral Immunity: Antibody Mediated Neutralization, Opsonization, and Complement Activation'. This presentation describes the topic on This lecture delves into the core effector mechanisms of humoral immunity, including antibody-mediated neutralization, opsonization, complement activation, and IgA-mediated protection. It also discusses how exaggerated complement activation can lead to disease and explains the concept of passive immunity in newborns.. The key topics included in this slideshow are humoral immunity, antibody-mediated neutralization, opsonization, complement activation, IgA-mediated protection, passive immunity, newborns, Jason Cyster,. Download this presentation absolutely free.
Presentation Transcript
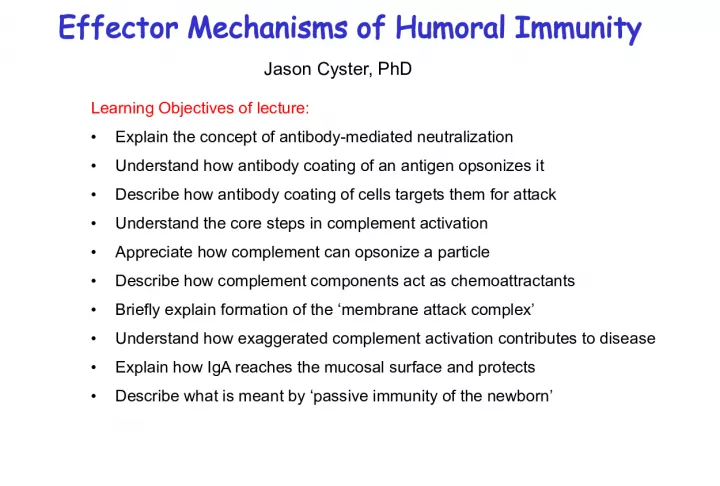
1. Effector Mechanisms of Humoral Immunity Learning Objectives of lecture: Explain the concept of antibody-mediated neutralization Understand how antibody coating of an antigen opsonizes it Describe how antibody coating of cells targets them for attack Understand the core steps in complement activation Appreciate how complement can opsonize a particle Describe how complement components act as chemoattractants Briefly explain formation of the membrane attack complex Understand how exaggerated complement activation contributes to disease Explain how IgA reaches the mucosal surface and protects Describe what is meant by passive immunity of the newborn Jason Cyster, PhD
2. Features of primary and secondary antibody responses
3. 5 Different Antibody Classes -> When B cells switch heavy chain, they keep the same Variable domain (and same light chain) and thus the same antigen binding specificity -> the different heavy chains confer different effector functions
4. Antibody Structure (reminder) Domains at ends of heavy chain and light chain are highly variable and responsible for binding antigen - the gene segments encoding these domains are mutated in GC B cells during antibody affinity maturation
5. The effector functions of antibodies
6. -> antibody responses can be long-lasting can exist for years after the initial encounter or vaccination (e.g. ~10 years for tetanus toxoid vaccination)
7. Antibody-mediated opsonization and phagocytosis of microbes Therapeutic antibodies such as Rituxan (against B cell surface molecule CD20) also utilize this pathway to promote clearance of targeted (cancer) cells. Phagocytic cells (macrophages, neutrophils) express activating Fc receptors - when crosslinked they transmit signals that promote engulfment and increased bactericidal activity.
8. Antibody-dependent cellular cytotoxicity (ADCC)
9. Complement opsonizes antigens for phagocytosis and can promote direct lysis of some bacteria C activation occurs in mulitple diseases e.g. glomerulonephritis, lupus, autoimmune heart disease, RA, age- related macular degeneration, Alzheimers disease All the C components pre-exist in an inactive form in the blood (mostly made in the liver); C3 is the most abundant Enzymatic (proteolytic) cascade - initial signal strongly amplified When a C component is cleaved, the larger b fragment is chemically labile and becomes covalently bound to nearby surfaces; the smaller a fragment is soluble and can diffuse away The Complement (C) System
10. Lectin Pathway Classical Pathway Alternative Pathway Mannose poly- saccharide & MBL Antibody/Antigen Complex & C1 Microbial surface- bound C3b Various complement components activated to generate C3 C3b + C3a C3 convertase (protease) Activation of Complement (C) MBL is C1-like but activated by binding Mannose-rich polysaccharides
11. IgM or IgG antibody Early steps of complement activation b b Note: because C3 is very abundant, once a C3 convertase forms, lots of C3b is generated and becomes covalently bound to nearby surfaces
12. Early steps of complement activation Classical Pathway : activation of the endogenous protease activity of C1 following binding to antigen- bound IgM or IgG leads to generation of a protease that cleaves C3 (a C3 convertase) Alternative pathway : a low level of C3 is spontaneously activated; when this occurs near a microbial surface the active C3 (C3b) can bind and then interact with components of the alternative pathway, leading again to formation of a C3 convertase
13. The late steps of complement activation C9 -> Gram negative organisms have a thin peptidoglycan layer and are the most sensitive to the MAC (e.g. Neisseria ) Key point: C5b catalyzes formation of the Membrane Attack Complex
14. Lectin Pathway Classical Pathway Alternative Pathway Mannose poly- saccharide & MBL Antibody/Antigen Complex & C1 Microbial surface- bound C3b Various complement components activated to generate C3 C3b + C3a C3 convertase (protease) C3b decorates surface; some C3b forms a C5 convertase, generating C5a and C5b; C5b causes formation of the Membrane Attack Complex (C5-C9) Activation of Complement (C) MBL is C1-like but activated by binding Mannose-rich polysaccharides Note: this slide summarizes all the core components of the C cascade that are useful to remember
15. The biological functions of complement C5a, C3a (and C4a) also known as Anaphylatoxins -> induce mast cell and basophil mediator release (when occurring systemically, can cause anaphylactic shock)
16. Regulation of complement (C) activation Host cells express membrane anchored C regulatory proteins that inactivate complement when it deposits on a host cell o Some of these proteins are linked to the membrane via a glycosylphosphatidylinosital (GPI) anchor Plasma contains soluble C1-Inhibitor (C1-INH) protein that limits the extent of C activation
17. Complement in disease Complement overactivity: 1) Immune complex glomerulonephritis damage caused by Antigen-Ab complexes deposited in glomerular basement membrane activating C and recruiting and activating neutrophils Strep pyogenes can cause acute glomerulonephritis (AGN) 2) Hereditary angioedema (deficiency of C1-INH) severe attacks of edema because the cascade is more easily activated and a lot of C3a, C5a are made 3) Paroxysmal nocturnal hemoglobulinuria (deficiency of GPI anchored membrane proteins - includes several C regulatory proteins) increased autologous red cell lysis Nocturnal because breathing patterns at night alter oxygen content of blood; leads to altered properties of RBC that make them more sensitive to lysis Complement deficiency C3 - increased risk of infection by many types of bacteria C5, C6, C7, or C8 - increased risk of Neisseria infections
18. The poly-Ig receptor is a special Fc receptor that binds dimeric IgA The process of transporting IgA across the cell is known as transcytosis The IgA released into the gut lumen remains associated with part of the poly-Ig receptor (known as the secretory component ) and this provides protection against proteolysis by gut proteases
19. Neonatal Immunity Maternal IgG is transported by the neonatal Fc receptor (FcRn) across the placenta to the fetus from colostrum across the newborn gut epithelium Colostrum is the protein-rich fluid secreted by the early postnatal mammary gland Confers passive immunity in the newborn The duration of protection is 3-4 months (or ~5 IgG half-lives) explains the high incidence of disease after this period by bacteria such as Haemophilus influenzae Human milk contains IgA provides some protection against gut pathogens Neonatal protection is only as good as the titer of IgG (and IgA) in the mother against the specific organism Fraction of adult level of serum immunoglobulins 100 passively transferred maternal IgG
20. IgG half-life FcRn is also present in the adult and involved in protecting IgG from degradation Accounts for the long (3 week) half-life of IgG compared to other Ig isotypes Therapeutic agents that are fused to IgG Fc regions take advantage of this property e.g. Enbrel (TNFR-Fc)
21. Evasion of humoral immunity by microbes Many viruses and bacteria mutate their antigen surface molecules such that they are no longer recognized by the existing antibody -> basis for existence of multiple serotypes of some pathogens (e.g. rhinoviruses, Salmonella enterica , Strep. pneumoniae ) -> population builds up immunity to some serotypes and remains susceptible to the others Some viruses have only a single serotype (e.g. measles, mumps) and this is the basis for the success of the vaccine
22. Ig Heavy chain class (isotype) switching Neutralization Neutralization Neutralization Eosinophil and
23. Vaccines Most vaccines work by inducing neutralizing Abs attenuated forms of microbes (treated to abolish infectivity and pathogenicity, but retain antigenicity) are most effective route of administration important e.g. oral administration of Polio vaccine ensures generation of neutralizing IgA immunization with inactivated microbial toxins generates toxin neutralizing antibodies Led to world-wide eradication of Small Pox May soon (?) achieve eradication of Polio Many vaccines still needed - HIV, Malaria, Schistosomes, Mtb etc
24. Classification of Licensed Vaccines This table is included just for interest Awareness of vaccines currently in use and the impact they have had on human health helps highlight the importance of understanding how to induce strong humoral immune responses