Empirical Formula
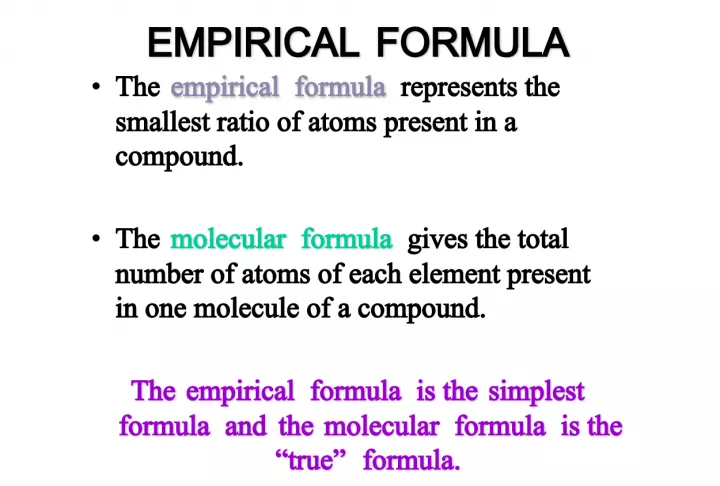

The empirical formula represents the smallest ratio of atoms present in a compound. It is a chemical formula that shows the proportion of elements in a compound, reduced to the simplest ratio possible.
- Uploaded on | 6 Views
-
 amandalahti
amandalahti
About Empirical Formula
PowerPoint presentation about 'Empirical Formula'. This presentation describes the topic on The empirical formula represents the smallest ratio of atoms present in a compound. It is a chemical formula that shows the proportion of elements in a compound, reduced to the simplest ratio possible.. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
1. EMPIRICAL FORMULA EMPIRICAL FORMULA empirical formula • The empirical formula represents the smallest ratio of atoms present in a compound. molecular formula • The molecular formula gives the total number of atoms of each element present in one molecule of a compound. The empirical formula is the simplest formula and the molecular formula is the “true” formula. The empirical formula is the simplest formula and the molecular formula is the “true” formula.
2. EMPIRICAL FORMULA EMPIRICAL FORMULA Assume 100g sample Calculate mole ratio Use Atomic Masses Mass % of Mass % of elements elements Grams of Grams of each each element element Moles of Moles of each each element element Empirical Empirical Formula Formula
3. EMPIRICAL FORMULA EMPIRICAL FORMULA Step 1: If given the % composition, assume a 100g sample then convert % to grams. Step 2: Use the atomic masses to convert grams to moles. Step 3: Divide the moles of each element by the SMALLEST mole fraction. Step 4: The results from step 3 should be a whole number, if not, make it so by multiplying by a common factor.
4. EMPIRICAL FORMULA EMPIRICAL FORMULA 1. Calculate the empirical formula from a sample containing 43.4% Na, 11.3% C, and 45.3% O. smallest smallest 43.4% 43.4 g Na (1 mole / 23 g/mol) =1.887 moles Na 11.3% 11.3 g C (1 mole / 12 g/mol) = 0.9417 moles C 45.3% 45.3 g O (1 mole / 16 g/mol) = 2.831 moles O 1.887/0.9417 =2.00 Na 2.831/0.9417 = 3.00 O . 9417/.9417 = 1.00 C Empirical Formula = Na 2 CO 3 Empirical Formula = Na 2 CO 3
5. EMPIRICAL FORMULA EMPIRICAL FORMULA . 2. When 8.00 g of calcium metal is heated in air, 11.20 g of metal oxide is formed. Calculate the empirical formula . According to the Law of Conservation of mass, 11.20 g Product - 8.00 g Ca = 3.20 g Oxygen (reactive part of air) smallest smallest 8.00 g Ca (1 mole / 40 g/mol) = 0.200 moles Ca 3.20 g O (1 mole / 16 g/mol) = 0.200 moles O 0.200 / 0.200 = 1 Empirical Formula = CaO Empirical Formula = CaO
6. EMPIRICAL FORMULA EMPIRICAL FORMULA 3. A compound was found to have a composition of 33.0 % Sr, 26.8 % Cl, and 40.2 % water. Calculate the empirical formula of this hydrate. smallest smallest 33.0% 33.0 g Sr (1 mole/87.6 g/mol) = 0.3767 moles Sr 26.8% 26.8 g Cl (1 mole/35.45 g/mol) = 0.7560 moles Cl 40.2% 40.2 g H 2 O (1 mole/18.0g/mol) = 2.233 moles H 2 O 0.7560 / 0.3767 = 2 Cl 2.233 / 0.3767 = 5.9 = 6 H 2 O Empirical Formula = SrCl 2 . 6 H 2 O Empirical Formula = SrCl 2 . 6 H 2 O
7. EMPIRICAL FORMULA & Molecular Formula EMPIRICAL FORMULA & Molecular Formula 4. smallest 4. Propylene contains 14.3 % H, 85.7% C, and has a molar mass of 42.0 g/mol. What is its molecular formula? smallest 14.3% 14.3 g H (1 mole/1.01 g/mol) = 14.19 moles H 85.7% 85.7 g C (1 mole/12.01 g/mol) = 7.142 moles C 14.19 / 7.142 = 1.987 = 2 H Empirical Formula = CH 2 Empirical Formula = CH 2 Molar mass / empirical mass = multipier Molar mass / empirical mass = multipier (42.0 g/mol / 14.0 g/mol) = 3 3 x CH 2 becomes the molecular formula C 3 H 6
8. PRACTICE PROBLEM #12 ______ 1. Which contains the larger number of MOLES of atoms? a) 125.0 g KCl b) 25.0 g CaSO 4 c) 17.0 g of N 2 ______ 2. What is the empirical formula of the compound whose composition is 39.7% K, 27.8% Mn, and 32.5% O? ______ 3. Determine the empirical formula of a compound that contains 89.7 % bismuth and 10.3 % oxygen. ______ 4. Write the molecular formula for a compound that contains 54.5 % C, 9.1% H, and 36.4 % O and has a molar mass of 132 amu? A K 2 MnO 4 Bi 2 O 3 C 6 H 12 O 3
9. GROUP STUDY PROBLEM #12 ______ 1. Which contains the larger number of MOLES of atoms? a) 125.0 g HBr b) 25.0 g C 6 H 11 O 6 c) 17.0 g of Br 2 ______ 2. A sample of a compound weighing 4.18 g contains 1.67 g of sulfur and the rest is oxygen. What is the empirical formula? ______ 3. What is the empirical formula of the compound whose composition is 28.7% K, 1.4% H, 22.8 % P, and 47.1% O? ______ 4. A compound contains 92.3% C and 7.7% H and has a molar mass of 78.0 g/mol. Determine the molecular formula.