Minimum Work for Separation Process

Exploring thermodynamics of separation, the study determines the minimum work required to separate a mixture into its pure components in practical applications like mining, desalination, and material purification.
- Uploaded on | 2 Views
-
 ashton
ashton
About Minimum Work for Separation Process
PowerPoint presentation about 'Minimum Work for Separation Process'. This presentation describes the topic on Exploring thermodynamics of separation, the study determines the minimum work required to separate a mixture into its pure components in practical applications like mining, desalination, and material purification.. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
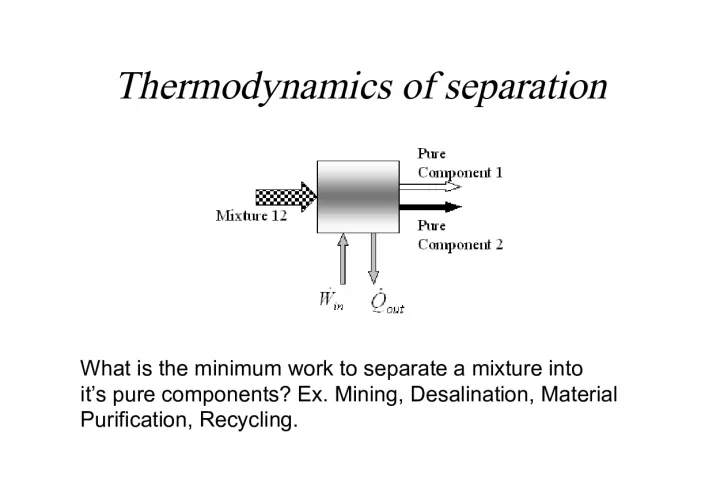
1. Thermodynamics of separation What is the minimum work to separate a mixture into its pure components? Ex. Mining, Desalination, Material Purification, Recycling.
2. Balance Eqns for Mass, Energy & Entropy S irr
3. Minimum Work of Separation
4. Gibbs Free Energy of Mixing* g o mix = h o mix T 0 s o mix . g o mix T 0 s mix = T 0 ( s 12 x 1 s 1 x 2 s 2 ) For non-interacting molecules entropy can dominate often resulting in a negative Gibbs Free Energy and hence spontaneous mixing. I.e. g o mix < 0 * at standard conditions
5. S = k ln Boltzmanns entropy equation How many ways can r atoms be positioned in a lattice with n locations?
6. w min = T 0 s mix = k T 0 ( ln 12 ) Ex. 4 atoms in 8 locations
7. Using Stirlings Approximation Where x is mol fraction r/n , and R = k N avo ln N! = N ln N - N
8. Multi-component System
9. Separation
10. Extraction
11. Separation Examples From the atmosphere From the Ocean Solutions Polymer Water based Liquid metals (activity coef)
12. The minimum work to separate O 2 from the atmosphere In wet air you get 3.97 kJ/mol : compare with Szargut Table from the EngineeringToolBox.com
14. energy requirements for mining and milling, possible future trends Chapman and Roberts p 113 & 116 underground ~ 1000/g (MJ/t metal) open pit ~ 400/g (MJ/t metal)
15. Sherwood plot showing the relationship between the concentration of a target material in a feed stream and the market value of (or cost to remove) the target material [Grbler 1998].
16. Exergy of a Mixture
17. CRUST at T o , p o Ore value at mine Pure ore (e.g. Fe 2 O 3 ) Pure metal Metal alloy Mixing in product Mixing in waste stream Further mixing and corrosion Exergy Purification Stages Recycle to pure metal Theoretical Exergy Values for a metal extracted from the earth s crust shown at various stages of a product life cycle (not to scale)