Precipitation Reactions: Understanding Double Replacement Reactions

Precipitation reactions occur when two compounds in aqueous solution exchange ions to form two new compounds - AX, BY, AY, BX. Typically, one of these
- Uploaded on | 1 Views
-
 frederik
frederik
About Precipitation Reactions: Understanding Double Replacement Reactions
PowerPoint presentation about 'Precipitation Reactions: Understanding Double Replacement Reactions'. This presentation describes the topic on Precipitation reactions occur when two compounds in aqueous solution exchange ions to form two new compounds - AX, BY, AY, BX. Typically, one of these. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
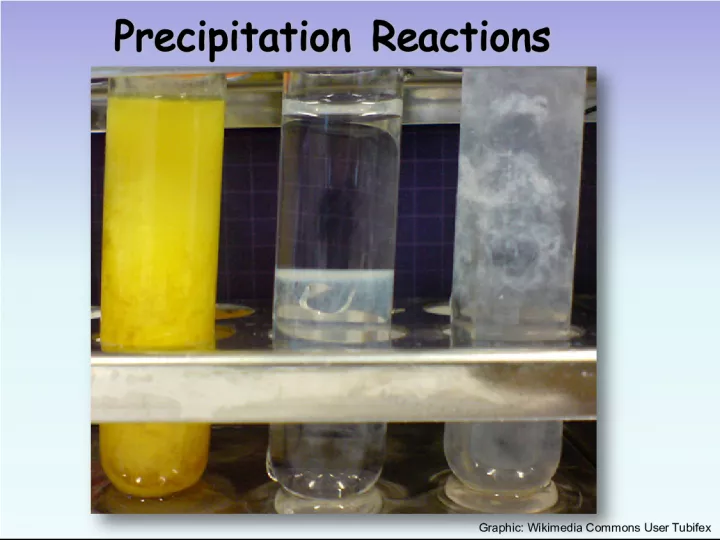
Slide1Precipitation Reactions Precipitation Reactions Graphic: Wikimedia Commons User Tubifex
Slide2Double Replacement ReactionsThe ions of two compounds exchange places in an aqueous solution to form two new compounds. AX + BY AY + BX One of the compounds formed is usually a precipitate (an insoluble solid), an insoluble gas that bubbles out of solution, or a molecular compound, usually water .
Slide3Double replacement forming a precipitate… Pb(NO 3 ) 2 (aq) + 2KI(aq) PbI 2 (s) + 2KNO 3 (aq) Pb 2+ (aq) + 2 NO 3 - (aq) + 2 K + (aq) +2 I - (aq) PbI 2 (s) + 2K + (aq) + 2 NO 3 - (aq) Pb 2+ (aq) + 2 I - (aq) PbI 2 (s) Pb 2+ (aq) + 2 I - (aq) PbI 2 (s) Double replacement (ionic) equation Complete ionic equation shows compounds as aqueous ions Net ionic equation eliminates the spectator ions Lead(II) nitrate + potassium iodide lead(II) iodide + potassium nitrate
Slide4Solubility Rules – AP Chemistry Solubility Rules – AP Chemistry All sodium, potassium, ammonium, and nitrate salts are soluble in water. Memorization of other “solubility rules” is beyond the scope of this course and the AP Exam. Therefore, the following slides are only for your amusement, and will not be tested
Slide5Solubility Rules – Mostly Soluble Solubility Rules – Mostly Soluble
Slide6Solubility Rules – Mostly Insoluble Solubility Rules – Mostly Insoluble
Slide7SolubilityChart: Common salts at 25 C Solubility Chart: Common salts at 25 C S = Soluble I = Insoluble P = Partially Soluble X = Other