Gene-environment interactions in dyslipidemia and cardiovascular disease

This article discusses how genetics and environmental factors contribute to dyslipidemia and cardiovascular disease, including co-dominant and recessive mutations, polymorphisms, and genome-wide association studies.
- Uploaded on | 1 Views
-
 gabrielveum
gabrielveum
About Gene-environment interactions in dyslipidemia and cardiovascular disease
PowerPoint presentation about 'Gene-environment interactions in dyslipidemia and cardiovascular disease'. This presentation describes the topic on This article discusses how genetics and environmental factors contribute to dyslipidemia and cardiovascular disease, including co-dominant and recessive mutations, polymorphisms, and genome-wide association studies.. The key topics included in this slideshow are genetic dyslipidemia, CVD risk factors, lipid metabolism, gene-environment interactions, genome-wide association studies,. Download this presentation absolutely free.
Presentation Transcript
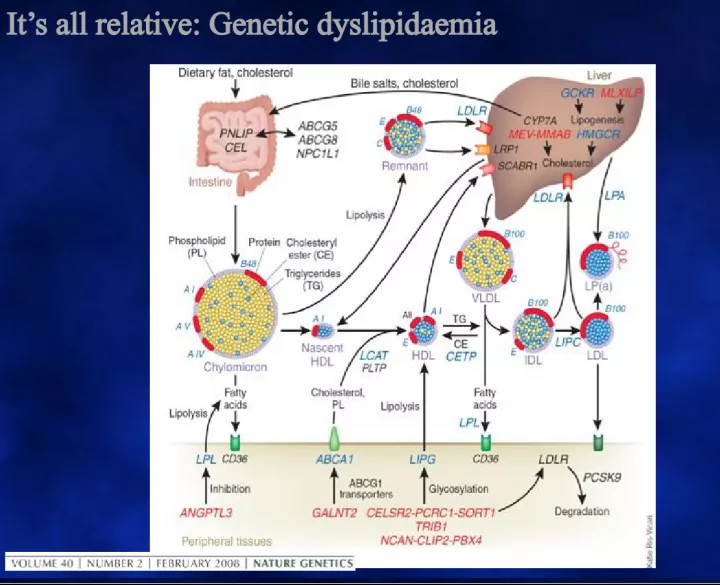
1. Its all relative: Genetic dyslipidaemia
2. CVD risk factors, and especially lipid metabolism, exemplify gene / environment interactions Mainly genetic Co-dominant mutations: Either genetic allele affected Recessive mutations: Both genetic alleles affected Polymorphisms and SNPs: A lleles consistent with normal and markers in proximity to significant genetic effects. Genome-wide association studies (GWAS). Mainly environmental or secondary to other disorders.
3. A missing piece of the genetic puzzle Clinical Effect Gene Prevalence Common genes with small effect Autosomal dominant Autosomal recessive Uncommon genes with large effect Polymorphisms Pathogenic mutations
4. Genetic hyper and hypo - cholesterolaemia Dominant Monogenic Hypercholesterolaemia Familial hypercholesterolemia (FH) Familial defective apo-100 (FDB-100) PCSK9 gain-of-function (FH-3) Hyperalphalipoproteinaemia (CETP deficiency, non-atherogenic?) Recessive Monogenic Hypercholesterolaemia Autosomal recessive hypercholesterolemia (ARH) Lysosomal acid lipase deficiency: Wolmans disease and Cholesterol ester storage disease Dominant Monogenic LDL deficiency PCSK9 loss-of-function Recessive Monogenic LDL deficiency Abeta and hypobeta lipoproteinaemia Chyomicron retention disease
5. Familial Hypercholesterolaemia: What goes wrong? NORMAL FH
6. LPL LIVER LIVER INTESTINE INTESTINE B Chylomicrons CII LPL R E B CII LDL-R LRP -48 IDL B E VLDL B E CIII -100 B CIII HL B OxLDL O . AI AI AII E SR- B1 HDL Macrophage ABCA1 LCAT Other Tissues CE FC FH SR-A SR- B1 LDL AI Metabolic Defect in Familial Hypercholesterolemia DAVIGNON 2006
7. LPL LIVER LIVER INTESTINE INTESTINE B Chylomicrons CII LPL R E B CII LDL-R LRP -48 IDL B E VLDL B E CIII -100 B CIII HL B OxLDL O . AI Macrophage ABCA1 SR-A LDL AI AII E SR- B1 HDL LCAT Other Tissues CE FC SR- B1 AI Metabolic Defect in Familial Defective ApoB-100 FDB ApoB Defective apoB B DAVIGNON 2006
8. PCSK9 regulates the surface expression of LDLRs by targeting for lysosomal degradation 1. Qian YW, et al. J Lipid Res . 2007;48:1488-1498. 2. Horton JD, et al. J Lipid Res . 2009;50:S172-S177. 3. Zhang DW, et al. J Biol Chem. 2007;282:18602-18612.
9. Gain-of-Function Mutations in PCSK9 Cause Familial Hypercholesterolaemia (FH) Associated with: High serum LDL-C 2 Premature CHD and MI 2 In vitro testing in many identified mutations show decreased levels of LDLRs 3 *For a full list of ADH mutations, please see refer to Abifadel reference. 1. Abifadel M, et al. Hum Gen . 2009;30:520-529. 2. Horton JD, et al. J Lipid Res . 2009;50:S172-S177. 3. Cameron J, et al. Hum Mol Genet. 2006;15:1551-1558. PCSK9 Variant Population Clinical Characteristics D374Y British, Norwegian families, 1 Utah family Premature CHD Tendon xanthomas Severe hypercholesterolemia S127R French, South African, Norwegian families Tendon xanthomas; CHD, early MI, stroke R215H Norwegian family Brother died at 31 from MI; strong family history of CVD
10. What is FH? What does it cause? FH is Co-dominant mutation of genes affecting formation or function of the LDL-receptor This causes metabolic and clinical consequences including precocious cardiovascular disease (CVD) Metabolic Increased LDL, Reduced clearance of remnants including LDLs precursor, IDL. Increased Lp(a)? Reduced HDL? Clinical Dominant: 50% of each generation. Risk 50:50 Premature CHD, CVD and PVD Aortic stenosis Tendon xanthomas (11%) specific? Corneal arcus (27%) non-specific > 40y? Xanthelasmas (12%) nonspecific No signs highly sensitive FH IS NOT JUST HIGH CHOLESTEROL IN A PATIENT AND THEIR RELATIVE(S)
11. FH: Why is it important? 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 25-30 30-35 35-40 40-45 45-50 50-55 55-60 60-65 65-70 70-75 75-80 80-85 85-90 90+ Age Cumulative Probability of Clinical CAD Non-FH Women Non-FH Men FH Women FH Men MED PED Registry 2001.
12. Why FH matters: Prevalence and Impact Prevalence : 0.2 0.5% (1 : 200-500) Atherosclerosis 173:55-68 > 8 x 10 6 affected world-wide. Seminars in Vasc Med 4:87-92 > 40,000 Australians Equal numbers of unaffected relatives Up to 1:60 in local groups with founder effect. Detection rates 0 44% Worlds best 20-40% World average (including Australia) < 5% Impact: 5 10% of CHD events under age 60. J Lipid Res 34:269-77 CVD death in >80% of FH cases. Absolute CVD risk differs from general population models. High risk profile from birth, Interaction differs (smoking, gender) Circulation 97:1837-47 Risk algorithms underestimate risk Eur Heart J 19:A2-11 Missed and misdiagnosed Molecular Medicine meets Public Health
13. Clinical protocol Laboratory protocol Cascade Screening Clinical services Detecting index cases Genetic testing Management Diagnosis assessment Diagnostic criteria Children, Adolescents Adults Children, Adolescents Adults Process Optimal components Model of Care for FH Process LDL- Apheresis Case detection: Dutch Lipid Clinic Score?
14. Severe triglyceride elevation due to recessive impairment of lipoprotein lipase Chylomicrons persist after fasting, massive levels of TG. Homozygous deficiency of Lipoprotein Lipase (LPL) Homozygous deficiency of the cofactor for L PL, Apo CII Impaired transport of LPL to site of action (endothelium) due to homozygous defect in ANGPTL or GPIHBP Combined overproduction and undercatabolism of triglyceride-rich lipoproteins sufficient to saturate LPL, eg in Apo AV mutations.
15. LIVER LIVER INTESTINE INTESTINE B Chylomicrons CII LPL CR E B CII LDL-R LRP -48 IDL B E VLDL B E CIII -100 B LDL CIII HL B OxLDL O . Metabolic Defects affecting Lipoprotein Lipase Macrophage ABCA1 SR-A -48 Dietary fat TG T G TG ApoB-48 R LPL VLDLR AI DAVIGNON 2006 AI AII E SR- B1 HDL LCAT Other Tissues CE FC SR- B1 AI FFA + MG
16. AV INTESTINE INTESTINE B Chylomicrons CII LPL CR E B CII LRP -48 IDL B E TG rich VLDL B E CIII -100 B CIII HL B OxLDL O . SR- B1 Metabolic defects saturating in LPL, eg Apo AV mutations Macrophage ABCA1 -48 Sugar fat calories LPL AI Overproduction of VLDL sdLDL ADIPOSE TISSUE GP + FFA TG Normal or reduced VLDL catabolism LDLR LOX-1 CD-36 SR-A SR-PSOX HSL CETP TG CE AV AI AII E HDL LCAT Other Tissues CE FC SR- B1 VLDLR DAVIGNON 2006 AI
17. Normal Hypoalpha- lipoproteinemia Complete (FHC) or partial LPL deficiency associated with a secondary factor Complete LPL deficiency (FHC) Primary apoC- II deficiency Familial dysbeta- lipoproteinemia (type III) Hepatic lipase deficiency (Primary cause associated with a secondary factor) Apo B > 0.75 g/L Apo B < 0.75 g/L TC:Apo B > 6.2 TC:Apo B < 6.2 Familial hyperTG Partial LPL deficiency FH Polygenic FDB PCSK9 deficiency ARH deficiency CYP7A1 deficiency Hypoalphalipo- proteinemia FCH - Sitosterolemia Normal Chylo + VLDL Chylo Chylo + VLDL Remnants VLDL LDL VLDL + LDL NormoTG < 1.5 mmol/L TG:Apo B > 0.12 NormoApo B < 1.2 g/L TG:Apo B < 0.12 HyperTG > 1.5 mmol/L NomoTG > 1.5 mmol/L HyperApo B > 1.2 g/L Hyper TG > 1.5 mmol/L Primary Causes Algorithm for Diagnosis of Apo B Dyslipoproteinemias Algorithm for Diagnosis of Apo B Dyslipoproteinemias Abbreviations: apo, apolipoprotein; ARH, autosomal recessive hypercholesterolemia; CAPD, continuous ambulatory peritoneal dialysis; Chylo, chylomicrons; CP7A1, cytochrome P450 7A1; DM2, diabetes mellitus type 2; dysbeta; dysbetalipoproteinemia; FCH, familial combined hyperlipidemia; FDB, familial defective apoB; FH, familial hypercholesterolemia; FHC, familial hyperchylomicronemia; HAART, highly active antiretroviral therapy; LPL, lipoprotein lipase; PCOS, polycystic ovary syndrome; SLE, systemic lupus erythematosus; TC, total cholesterol; TG, triglyceride. de Graaf J et al. Nat Clin Pract Endocrinol Metab 2008;4:608- 18. Lipoproteins
18. Autosomal recessive disorders have revealed HDL metabolism A-I FC A-I ABCA1 Macrophage Rapid catabolism LCAT Nascent HDL CE FC Homozygous (?heterozygous) Apo AI deficiency: Atherogenic Tangiers Disease: ABC-AI deficiency: Atherogenic? LCAT Deficiency: Non-atherogenic? Apo A1 Milano: Anti-atherogenic? +
19. 100 50 0 30 40 50 60 70 80 Age ( years) Event Free Survival ( % ) apoAI (L178P) carriers (n=54) family controls (n=147) p = 0.008 Carriers of the ApoAI Leu 178 Pro Variant Are at Increased Risk of Developing CAD Hovingh K et al. J Amer Coll Cardiol 44:1429, 2004 ApoAI 50% HDL-C 63% 18.9 x CAD risk DAVIGNON 2006
20. Normal ApoAI and ApoAI MILANO Dimer 1 25 35 66 121 165 209 220 243 187 143 99 Lipid Binding In Vivo Catabolism LCAT Activation Cholesterol Efflux Receptor Binding AI Franceschini G Eur J Clin Invest 26; 733, 1996 AI m /AI m 1 1 243 243 173 173 s s DAVIGNON 2006
21. Role of apolipoprotein E Apo E: ligand for hepatic removal of remnants. Apo E knockout model is atherogenic Apo E2 has lower binding affinity (E4>E3>E2). E2:E2 only critical if lipids increase for other reasons. Other roles for Apo E (CNS lipid transport and neural repair). CR E E AI CHYLOMICRON CIII TG TG TG OOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOO OOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOOO LPL LRP AII C E E E AI CE HDL B 48 B 48 CII Lipolysis products C FC AI
22. SR-B1 CE CE FC FC AI AII E CE CE AI FC FC AI AII E CE CE HL CE CE Kidney AI HDL 3 HDL 2 HL CE CE E CE CE AI HDL 3 LCAT LCAT FC/PL FC/PL FC/PL FC/PL AII AI Metabolic Defects in Remnant Dyslipidaemia Apo E2 homozygosity plus apo B overproduction or Hepatic Lipase deficiency LIVER LIVER CELL CELL ABCA1 VLDL to Remn B E CE CE CETP Chylo to ChyloRe B E LPL PLTP INTESTINE INTESTINE LDL B HL Degradation (catabolic) Formation (anabolic) CE CE TG TG LDLR TG TG Ox LDL B LRP Modified from Deeb SS et al. J Lipid Res 44:1279, 2003
23. Unspecified hereditar y Dyslipoproteinemia Common: Lipoptotein (a) Rare: Familial Phytosterolemia DAVIGNON 2006 ABCG5 ABCG8 Micelle s Phytosterols Phytosterols Cholesterol Cholesterol Protease K V K IV Types 3-10 Apo(a) LDL Receptor Binding LDL Receptor Binding Site on apoB Site on apoB K IV Type 2 ApoB LDL particle K IV Type 1 C N -C -N DAVIGNON 2006
24. Genetic dyslipidaemias have motivated treatment discovery Clinical abnormalities represent real human problems Massive yield on research into genetic dyslipidaemia Familial Hypercholesterolaemia: Receptor mediated endocytosis Statins, PCSK9 antisense and Abs Familial Hyperchylomicronaemia: LPL gene therapy Apo A1 Milano: Synthetic HDL Deficiency of Apo B or MTP MTP inhibitors, Apo B antisense CETP Deficiency CETP inhibitors Apo E Valuable animal k/o model
25. AAS MASTERCLASS AAS MASTERCLASS 5-6 TH OCTOBER 2012 5-6 TH OCTOBER 2012
26. Referred to Specialist Llipid Clinic Hypercholesterolaemia Yellowish butterfly patterned lesion around her eyes On Pravastatin 20mg ON TC 13.7, LDL-c 11.6 mmol/L July 1997 Cycling accident at her home town Sustained minor soft tissue injury Noted to have xanthelasma around her eyes Lipid profile results: TC : 15.4 mmol/L LDL: 13.9 mmol/L Referred to Medical Clinic, home town Started Pravastatin 20 mg/ON 6 th August 1998 HISTORY Miss KM, 21year old Malay student nurse
27. Noted yellowish butterfly patterned lesion surrounding both eyes since primary school days Asymptomatic, well No h/o chest pain, palpitation, shortness of breath or poor effort tolerance No history of intermittent claudication or syncopal attacks. No h/o polyuria, polydipsia No h/o cold intolerance, lethargy or menstrual disturbance No past h/o jaundice, liver or renal diseases Not HT, DM No past surgical history HISTORY
28. - HISTORY The youngest out of 11 siblings 3 siblings died : eldest brother : died @ 43 years AMI (HC) elder brother (6 th ) : died @ 23 years AMI (HC, xanthelasma) eldest sister (2 nd ) : died @ 33 years - ?MI, ? SCD Parents - consanguinous marriage (first cousins), not known to have DM/HPT/CAD
29. - HISTORY Student nurse at a Teaching Hospital, KL Single Non smoker No history of alcohol intake Exercise - once weekly (jogging), 30minutes Normal diet, low fiber intake
30. Physical Examination Anthropometry: BMI : 22.1 Waist circumference : 61cm (<80cm) Waist-to-hip ratio: 0.71 (<0.85) PR: 72/min, regular, BP : 120/62 mmHg, DRNM Peripheral pulses present, carotid & renal bruit : absent Other systems: Normal
31. Xanthelasma
32. Corneal Arcus grading: 0 no arcus observable 1- upper or lower segments affected 2- both segments affected 3- both segments and just confluent 4- heavy confluent arcus (Wider et al, 1983) Right Eye Left Eye Grade 4 Grade 4 Corneal Arcus
33. Achilles tendon xanthomata Digital xanthomata
34. Test Result Reference range ** FSL Baseline (1997) 1 st visit to the SLC TC 15.4 13.7 < 5.7 mmol/L HDL-c 1.5 1.6 > 1.3 mmol/L LDL-c 13.9 11.6 <3.8 mmol/L Triglycerides 1.0 1.1 < 1.3 mmol/L On pravastatin 20 mg / ON x 1 year
35. Summary of KMs risk factors Positive risk factors: Negative risk factors: Markedly elevated TC & LDL levels Strong family history of premature CAD HDL > 1.3 mmol/L Non smoker Not hypertensive Not DM Not overweight/ obese Premenopausal female
36. Differential Diagnosis 1 Hypercholesterolaemia 2 Hypercholesterolaemia - TRO Familial hypercholesterolaemia (FH) Hypothyroidism Familial Defective ApoB100 Cholestasis PCSK9 gain-of-function mutation (FH-3) Nephrotic syndrome Polygenic hypercholesterolaemia
37. Tests Results Reference Ranges TFT fT4 11.78 9.10 23.8 nmol/L TSH 1.22 0.32- 5.00 Iu/L FPG Glucose 4.4 <6.1 mmol/L Renal Profile Sodium 134 135 150 mmol/L Potassium 4.2 3.5 5.0 mmol/L Urea 3.2 2.5 6.4 mmol/L Creatinine 66 44-80 umol/L
38. Tests Results Reference Ranges LFT Albumin 42 35-50 g/L Total Protein 84 67-88 g/L Bilirubin Total 16 < 23 umol/L ALT 17 <44 U/L ALP 89 32 104 U/L Creatine Kinase 42 24-135 U/L Lp(a) 0.58 <0.3g/L
39. OTHER INVESTIGATIONS. ECG normal Exercise stress test normal ECHO Normal, no evidence of aortic stenosis Carotid artery IMT normal, no evidence of atheromatous plaques
40. Question 1 What are the various criteria for the diagnosis of FH? Dutch Lipid Clinic Network diagnostic scoring Simon Broomes criteria MedPed criteria for FH NCEP ATPIII criteria
41. Question 2 According to the Dutch Lipid Clinic Network criteria, scoring for definite FH is: > 8 points 6 8 points 3 5 points
42. In determining the Dutch Lipid Clinic diagnostic scoring for FH, the following are taken into account: Baseline LDL-c concentration: Yes/ No Clinical history of premature CAD: Yes/ No Clinical history of premature cerebral or PVD: Yes/ No Tendon xanthoma(ta) in the patient: Yes/ No Premature corneal arcus in the patient: Yes/ No Family history of hypercholesterolaemia: Yes/ No Family history of premature CAD/PVD in 1 st degree relatives: Yes/ No Family history of tendon xanthomata and/or corneal arcus in 1 st degree relatives: Yes/ No Question 3
43. Dutch Lipid Clinic Diagnostic scoring for FH Criteria: 8 points - DNA Mutation, or LDL-C > 8.5mmol/L 6 points - Tendon xanthomas 5 points - LDL-C 6.5 8.4mmol/L 4 points - premature corneal arcus < 45 yrs 3 points - LDL 5.0 6.4mmol/L 2 points - 1st degree relative with xanthomas or premature CA or childhood LDL > 95 th percentile, or personal premature CAD 1 point - 1st deg relative with premature CAD/ vascular dis or LDL > 95 th percentile, or personal history of premature cerebral or PVD, or LDL-c 4.0 4.9mmol/L Criteria: Family history: 1st degree relative with (a) premature CAD or vascular dis (men<55yrs, women<60yrs) OR (b) LDL > 95 th percentile, in - 1 point and / or 1st degree relative with tendon xanthomata and/or corneal arcus OR childhood (<18yrs) LDL > 95 th percentile - 2 points Clinical history Patient with premature CAD (men<55, women <60yrs) - 2 points Patient with premature cerebral or PVD (men<55, women <60yrs) -1 point Physical Examination - patient Tendon xanthomas - 6 points premature arcus - 4 points Lab Analysis LDL-c >8.5mmol/> - 8 points LDL-c 6.5 8.4 - 5 LDL-c 5.0 - 6.4 - 3 LDL-c 4.0 4.9 - 1 DNA analysis Functional DNA Mutation - 8 points Definite: > 8 points, Probable: 6 8 points, Possible FH: 3-5
44. US MedPed Criteria vs Simon Broome criteria for the dx of FH Total cholesterol cutpoints (mmol/L) 1st-degree vs 2nd-degree vs 3rd-degree relatives with FH vs General population Age (years) 1 st -degree 2 nd degree 3 rd degree General population <20 5.7 5.9 6.2 7.0 2029 6.2 6.5 6.7 7.5 3039 7.0 7.2 7.5 8.8 40 7.5 7.8 8.0 9.3 FH is diagnosed if TC levels exceed the cutpoint Key: FH = familial hypercholesterolaemia
45. Simon Broome Criteria - Diagnosis of FH Criteria Description (A) TC > 7.5 mmol/L in adults or TC > 6.7 mmol/L in children <16 years, or LDL-c > 4.9 mmol/L in adults or > 4.0 mmol/L in children (B) Tendon xanthomas in the patient, or a first-degree or second- degree relative (C ) DNA-based evidence of mutation in the LDLR, or apo- B100 or PCSK9 gene (D) Family history of premature CHD (age <50 years in a second-degree relative or <60 years in a first-degree relative) (E) Family history of raised TC > 7.5 mmol/L in a first- or second- degree relative, or >6.7mmol/L in child, brother or sister <16yrs of age Diagnosis Definite FH diagnosis requires either A + B or A + C Possible FH diagnosis requires either A +D or A + E Key: FH = familial hypercholesterolaemia
46. Hypercholesterolaemia (LDL-c 13.9mmol/L) Xanthomata, corneal arcus, xanthelasma No evidence of personal CAD Strong family history of premature CAD Positive family history of HC 1 st and 2 nd degree relative Consanguity Ruled out secondary causes of HC Summary
47. CLINICAL DIAGNOSIS DEFINITE FAMILIAL HYPERCHOLESTROLEMIA
48. Question 4: Should risk estimation tools eg Framingham Risk Scoring be used for her? YES NO
49. CHD Risk Stratification CHD estimation tools such as those based on the Framingham risk scoring SHOULD NOT be used because people with FH are already at high risk of premature CHD. Heterozygous FH has >50% risk of CHD in men by the age of 50years, >30% in women by the age of 60years NICE Clinical Guideline 2008- Identification and Mx of FH 2.5.1 Individuals with FH are at high CHD risk . The 10-year CHD risk in the FH patient is not adequately predicted by any conventional risk assessment tools. Therefore, assessment of 10- year risk is not recommended.
50. Management Statin therapy - continued, titrated Add-on lipid lowering Lifestyle modification Referred to dietician Referred to : - Cardiology - Plastic surgery Family tracing, cascade screening and counselling
51. Medication TC (mmol/L) TG HDL LDL Baseline, statin naive 15.4 1.0 1.5 13.9 Pravastatin 20 mg /ON 13.7 1.1 1.6 11.6 Pravastatin 20 mg/ON 11.9 1.1 1.0 10.4 Simvastatin 30 mg/ ON 13.1 1.1 1.4 11.3 Atorvastatin 80 mg/ON 15.3 1.4 1.2 13.5 Atorvastatin 80 mg/ON 11.7 0.7 1.26 10.1 Atorvastatin 80 mg/ON 11.3 0.7 1.1 9.8 Atorvastatin 80 mg/ON 11.9 0.6 1.4 10.2 Atorvastatin 80 mg/ON 11.5 1.1 1.2 9.8 Simvastatin 80mg & Ezetimibe 10mg/ ON 8.9 1.2 1.1 7.3
52. Alternative or add-on lipid lowering therapy Ezetimide Bile acid binding resin Fibric acid derivatives Nicotinic acid LDL-apharesis
53. LIFESTYLE MODIFICATION Lifestyle modification - reduce LDL-c and other coronary RFs Modest, variable degree of LDL reduction 10% Diet low fat <30% calories, saturated fat < 10% of calories, cholesterol < 200mg/day, fibre 10-25g/day, caloric deficit 300- 500kcal/day Plant sterol esters or plant stanol esters 2g/day Physical activity 30min/day, moderate intensity, at least 5days per week Ideal weight BMI < 23 (Asian) Smoking Alcohol intake HT, DM
54. 1 3 2 4 5 6 7 8 9 10 11 6 3 62 Father Mother FAMILY TREE - 1998 4 3 33 4 0 3 9 3 8 2 3 2 3 3 2 3 3 2 6 2 1 HC CAD+ SD ?MI HC Xanthoma HC Xanthoma HC Xanthoma HC Xanthelasma Xanthelasma CAD+ HC Those found to have HC following family screening
55. 1 3 2 4 5 6 7 8 9 10 11 TC : 8.1 LDL : 5.7 TC : 8.7 LDL : 6.7 TC : 10.1 LDL : 6.7 TC : 8.7 LDL : 6.5 TC : 8.7 LDL : 6.5 TC : 7.7 LDL : 4.6 TC : 8.0 LDL : 6.4 TC : 16.2 LDL : 14.8 TC : 6.5 LDL : 4.6 TC : 7.0 LDL : 5.2 TC : 8.1 LDL : 5.7 TC : 4.9 LDL : 2.6 TC : 15.3 LDL : 13.9 Father Mother Family Screening ( Lipid Screening) - 1999 Affected with FH Died TC : 6.1 LDL : 4.4 FH with known mutation 43 33 40 39 38 23 33 32 26 23 21
56. Genetic testing was performed on KM and her family members Question 5: What genes have been implicated in FH? LDL Receptor Lipoprotein lipase Apo B100 Apo CII PCSK9
57. Low Density Protein Receptor (LDLR) Gene Cytogenetic Location: 19p13.2 Size: 44,469 bases Mosaic protein of ~840 amino acids (after removal of signal peptide); Molecular weight 95,376 Dalton
58. Made up of a number of functionally distinct domains that can function independently of each other. Ex 1 contains a signal sequence that localizes the receptor to the ER for transport to the cell surface Ex 2-6 code the ligand binding region Ex 7-14 code the EGFP domain Ex 15 codes the oligosaccharide rich region Ex 16 (and some of 17) code the membrane spanning region Ex 18 (with the rest of 17) code the cytosolic domain. LDLR PROTEIN
59. PCR PRODUCT ANALYSIS OF LDLR EXON 5 DNA CHIP CAPILLARY GEL ELECTROPHORESIS 182bp Amplification of Exon 5 of LDLR gene by PCR. PCR products were analysed on an automated on-chip capillary gel electrophoresis (Agilent Bioanalyser). Lanes from left to right; 1-Kb ladder, Negative control sample, patients samples and normal control sample. The green band represent lower marker and purple band represent upper marker. The PCR products were detected at 182bp.
60. DHPLC RESULTS LDLR Exon 5 g.763T>A g.763T>A Homoduplex peak of NC sample (wild type sample) Heteroduplex peaks of FH patients with variant in exon 5 Heteroduplex peaks of FH patients with variant in exon 5 Mutation Screening by DHPLC Wash peak The DHPLC chromatogram profiles of FH patients showed presence of heteroduplex peaks eluted at 5.2 mins and 5.8 mins at denaturing temperature of 62.8 0 C. The presence of heteroduplex peaks were suggestive of disease-causing variants and subjected to DNA sequencing to confirm the variants.
61. DNA SEQUENCING RESULTS FH Patient ID Affecte d LDLR regions DNA Sequence Description of variant and effect KM, SFM & WC (3 members of a Malay Family) Exon 5 Reference :GCCGGCAG T GTGACCG Variant :GCCGGCAG A GTGACCG g.763T>A; Cysteine (C) to Serine (S); Homozygous and Heterozygous mutation C234S NYK Exon 9 Reference : CTTCACCAAC C GGCACG Variant : CTTCACCAAC T GGCACG g.1216C>T; Arginine (R) to Tryptophan (W), Heterozygous mutation R385W
62. DNA SEQUENCING RESULTS Affected LDLR region DNA Sequence Description of variant and effect Exon 5 Reference: GCCGGCAG T GTGACCG Variant : GCCGGCAG A GTGACCG g.763T>A; Cysteine(C) to Serine (S) at codon 234. Identified as homozygous mutation C234S. Reference sequence from normal control DNA sequence of Homozygous FH patient Homozygous: Substitution T>A at nucleotide position 763 (g.763T>A)
63. DNA SEQUENCING RESULTS Affected LDLR region DNA Sequence Description of variant and effect Exon 5 Reference: GCCGGCAG T GTGACCG Variant : GCCGGCAG A GTGACCG g.763T>A; Cysteine(C) to Serine (S) at codon 234. Identified as heterozygous mutation C234S. Reference sequence from normal control DNA sequence of Heterozygous FH patient Heterozygous: Substitution T>A at nucleotide position 763 (g.763T>A)
64. KM is now married with 2 children Questions 5: What is the probablity that her children may have FH with every pregnancy? 50% 25% Questions 6: When should diagnostic tests be done for her children? At birth By the age of 10 years or earliest opportunity thereafter
65. AD, one affected parent 1:2 (50%) chance of heterozygous with every pregnancy In children with one affected parent, the following Dx-tic tests should be done by the age of 10yrs or at the earliest opportunity thereafter: - DNA test if the family mutation is known - LDL-c if the family mutation is not known. To exclude the Dx of FH, LDL-c should be repeated after puberty - LDL-c concentration changes at puberty to almost similar to that of adult concentrations Cascade testing combination of DNA testing and LDL-c to identify affected relatives In children at risk of homozygous FH (2 affected parents or presence of clinical signs eg cutaneous xanthomata), measure LDL-c before 5yrs of age or at the earliest opportunity thereafter. If LDL >11mmol/L, clinical Dx of homozygous FH should be considered NICE Clinical Guideline 2008- Identification and Mx of FH
66. Question 7: Who can be considered for LDL apheresis treatment? Adults and children/young people with Homozygous FH Heterozygous FH
67. LDL lowering apheresis Treatment of adults and children/ young adults with homozygous FH - In children with 2 affected parents - Presence of clinical signs eg cutaneous lipid deposits (xanthomata) - LDL-c should be measured before 5 yrs of age, or earliest opportunity thereafter - LDL-c > 13mmol/L (adults), > 11mmol/L (children/ young adults) consider clinical diagnosis of homozygous FH Exceptional cases of heterozygous FH progressive symptomatic CHD despite maximal tolerated lipid-modifying drug therapy, optimal medical and surgical therapy Specialist centre Cost implication
68. Question 8: What are the future lipid lowering therapy? Antisense oligonucleotides (ASO) to inhibit Apolipoprotein B production eg Mipomersen PCSK9 targeted therapy eg PCSK9 inhibitor Microsomal TG Transfer Protein Inhibitors eg lomitapide Thyroid Mimetics eg eprotirome Cholesterol Ester Transfer Protein (CETP) Inhibitors - anacetrapib, dalcetrapib
69. (1) ANTISENSE OLIGONUCLEOTIDES (ASO) MIPOMERSEN Subcutaneous 200mg 1x/wk phase 3 CT, LDL reduction lasted for 4/52 after last dose. No clinically relevant interactions wrt clearance of statins and ezetimide - impt as add-on therapy Yu et al Clin Pharmacokinet 2009;48:39-50 Homo FH (n=51) - Recent double blind CT, sc mipomersen 200mg/wk vs placebo x26wks 25% vs 3% LDL reduction, 27% reduction apoB, 21% reduction TC Raal FJ et al. Lancet 2010;375:998-1006 Hetero FH (n=124), CAD+ on max tolerated statins: 45% of these high risk subjects achieved target LDL of < 100mg/dL (2.6mmol/L) EAS Congress 2011
70. 2. PCSK9 TARGETED THERAPY Animal studies beneficial up to 80% LDL lowering, human studies not published yet Frank-Kamenetsky et al. Proc Natl Acad Sci USA 2008;105:11915-20 Chan JC et al. Proc Natl Acad Sci USA 2009;106:9820-5 Statins and fibrates induce increase PCSK9 expression, thus PCSK9 inhibition could induce robust LDL reduction as add-on therapy in FH
71. 3. MICROSOMAL TG TRANSFER PROTEIN (MTP) INHIBITORS MTP inhibition with BMS-201038 in homozygous FH (n=6), dose 1mg/kg/day - 50% LDL reduction but noted to induce hepatic steatosis Cuchel et al. New Engl J Med 2007;356:148-56 MTP inhibition in homozygous FH (n=10) , dose 60mg/D 44% LDL reduction; less steatosis Cuchel et al.Circulation 2009;120:S441 Ezetimibe 10mg vs MTP inhibition by lomitapide (5-10mg/ D x4/52) 20% vs 20-30% in a dose-dependant manner Combined therapy (ezetimide + lomitapide) similar but larger dose dependent LDL reduction (35-45%); SE: Mild ALT increase, diarrhoea Samaha et al. Nat Clin Pract Cardivasc Med 2009;2010:906-16 Potential attractive candidate for lipid lowering in FH patients if administered in lower doses
72. 4. THYROID MIMETICS Thyroid hormone analogues reduce LDL but associated with cardiac and bone related SEs More recently differential molecular mechanisms (cpds that act on TR-beta mainly expressed in the liver, do not affect TR-alpha expressed in brain and heart) eg eprotirome, selective TR beta agonists (under Ix) Statin + placebo/ eprotirome for 12/52 20-30% additional reduction of LDL; no SE on heart or bone Ladenson et al. Use of thyroid hormone analoque eprotirome in statin-treated dyslipidaemia. New Engl J Med 2012;362:906-16
73. 5. CETP (CHOLESTEROL ESTER TRANSFER PROTEIN) INHIBITORS X2 approaches to inhibit CETP (a) Vaccine - CETi-1 synthetic CETP peptide vaccine, immune response anti CETP- Abs Animal studies of this vaccine increase HDL, reduce aortic atherosclerosis but human studies poor response of autoAB production Rittershaus et al. Vaccine induced Abs inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis, ATVB 2000;20:2106-12 Davidson et al. The safety and immunogenicity of a CETP vaccine in healthy adults. Atherosclerosis 2003;169:113-20 (b) Small molecule CETP inhibitors eg Torcetrapib, anacetrapib, dalcetrapib - antagonize CETP activity by binding to it. RADIANCE I 800 FH pts, torce + atorva: No reduction in atherosclerosis (IMT) despite reduction in LDL (20%) and increase HDL (52%) Kasteleine JJ et al. Effect of torcetrapid on carotid atherosclerosis in FH. NEJM 2007;356: 1620-30
74. ILLUMINATE torce + atorva, prematurely terminated, unexpected increase in M&M exact mechanisms unclear ?torce induced increase BP Barter et al. Effects of tercetrapid in patients at high risk of coronary event, NEJM 2007;357:2109-22 Xie et al. Drug discovery using chemical systems biology:identidfication of the protein-ligand binding network to explain the side effects of CETP inhibitors. PLoS Comput Biol 2009;5:e1000387 2 other CETP inhibitors have no effect on BP molecule-specific off- target effect Anacetrapid and dalcetrapib phase 3 clinical trials , mild HC and pts with CVD risk, effective lipid modifiers Pending CV outcome trials (DAL-outcomes I and II and HPS3/REVEAL) CETP inhibition likely to benefit patients with FH 5. CETP (CHOLESTEROL ESTER TRANSFER PROTEIN) INHIBITORS Cont.
75. Summary A 21year old Malay female student nurse who presented with incidental xanthelasma and severe hypercholesterolaemia associated with a strong family h/o premature CAD, xanthomata and grade 4 corneal arcus. There was consanguity between her parents. She is slim, non-smoker, euglycaemic, normotensive and has good HDL- c levels (>1.3mmol/L). Family tracing was performed. Molecular analyses confirmed the presence of homozygous FH with exon 5, position 763 T>A mutation of the LDLR, coding for the LDL binding domain. The updates on the present and future management, and the molecular pathogenesis of FH have been discussed.
76. Learning Issues FH criteria for diagnosis High coronary risk category, should not use risk assessment tools LDL Receptor protein and gene Genetic testing when to test for children? Lifestyle modification Current treatment, targets Plasma apheresis when is it indicated? Future lipid lowering therapy