Understanding Alkalinity in Natural Waters


This article defines and explains alkalinity, the measure of water's ability to neutralize acids and resist changes in pH. It also identifies the three primary species that contribute to alkalinity in natural waters.
- Uploaded on | 0 Views
-
 kalliewelch
kalliewelch
About Understanding Alkalinity in Natural Waters
PowerPoint presentation about 'Understanding Alkalinity in Natural Waters'. This presentation describes the topic on This article defines and explains alkalinity, the measure of water's ability to neutralize acids and resist changes in pH. It also identifies the three primary species that contribute to alkalinity in natural waters.. The key topics included in this slideshow are alkalinity, pH, neutralize, hydroxyl ions, carbonate ions, bicarbonate ions,. Download this presentation absolutely free.
Presentation Transcript
1. Alkalinity Alkalinity CE 370 Lab CE 370 Lab
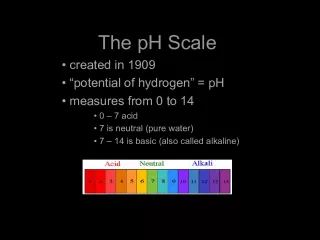
2. Definition Definition Alkalinity is defined as the measure of the water capacity to neutralize acids. In other words, the ability to resist the change in pH. Alkalinity is defined as the measure of the water capacity to neutralize acids. In other words, the ability to resist the change in pH.
3. Alkalinity of natural waters is primarily due to three species: Alkalinity of natural waters is primarily due to three species: OH (hydroxyl ions) OH (hydroxyl ions) CO 3 = (carbonate ions) CO 3 = (carbonate ions) HCO 3 (bicarbonate ions) HCO 3 (bicarbonate ions)
4. Importance of Alkalinity Importance of Alkalinity Alkalinity is involved (needed) in different water and wastewater treatment processes, such as: Alkalinity is involved (needed) in different water and wastewater treatment processes, such as: Chemical coagulation : Chemical coagulation : Calculation of the dose of the chemical coagulants depends on the Alkalinity of the water. Calculation of the dose of the chemical coagulants depends on the Alkalinity of the water. Al 2 (SO 4 ) 3 + 6H 2 O 2Al(OH) 3 + 6H + + 3SO 4 = Al 2 (SO 4 ) 3 + 6H 2 O 2Al(OH) 3 + 6H + + 3SO 4 = Water softening : Alkalinity is a major factor considered in calculating the lime and soda-ash requirements. Water softening : Alkalinity is a major factor considered in calculating the lime and soda-ash requirements. Corrosion Control : Alkalinity is an important parameter involving corrosion control calculations . Corrosion Control : Alkalinity is an important parameter involving corrosion control calculations .
5. Determination of the Different Species of Alkalinity Determination of the Different Species of Alkalinity First, we have to make two Alkalinity measurements: First, we have to make two Alkalinity measurements: Phenolphthalein Alkalinity (P): Alkalinity measured to P end point Phenolphthalein Alkalinity (P): Alkalinity measured to P end point Methyl Orange Alkalinity (M): Alkalinity measured to M end point Methyl Orange Alkalinity (M): Alkalinity measured to M end point Total Alkalinity (T) = P + M Total Alkalinity (T) = P + M
6. Second, we determine the species as the following: Second, we determine the species as the following: ( we assume that OH and HCO 3 do not exist together ) ( we assume that OH and HCO 3 do not exist together ) Possibilities: Possibilities: OH only OH only CO 3 = only CO 3 = only OH and CO 3 = OH and CO 3 = CO 3 = and HCO 3 CO 3 = and HCO 3 HCO 3 only HCO 3 only
7. Then: Then: Case 1 M = 0 P = T OH only Case 1 M = 0 P = T OH only Case 2 P = M P = T CO 3 = only Case 2 P = M P = T CO 3 = only Case 3 P > M P > T OH and CO 3 = Case 3 P > M P > T OH and CO 3 = OH = P M and M = T P OH = P M and M = T P Then, OH = P (T-P) = 2P T Then, OH = P (T-P) = 2P T And, CO 3 = = 2M = 2 (T - P) And, CO 3 = = 2M = 2 (T - P) Case 4 P < M P < T CO 3 = and HCO 3 Case 4 P < M P < T CO 3 = and HCO 3 CO 3 = = 2 P CO 3 = = 2 P HCO 3 = T - 2 P HCO 3 = T - 2 P Case 5 P = 0 T = M HCO 3 only Case 5 P = 0 T = M HCO 3 only HCO 3 = T HCO 3 = T
8. Expressing the Concentration of Alkalinity Expressing the Concentration of Alkalinity Alkalinity concentration is expressed as CaCO 3 for convenience to make it possible to add and subtract. Alkalinity concentration is expressed as CaCO 3 for convenience to make it possible to add and subtract. Conc. (mg/ l ) = N eq. wt. 1000 Conc. (mg/ l ) = N eq. wt. 1000 Conc. of Alkalinity = N Alk eq. wt. Alk 1000 Conc. of Alkalinity = N Alk eq. wt. Alk 1000 Conc. of Alkalinity (as CaCO 3 ) = N Alk eq. wt. CaCO3 1000 Conc. of Alkalinity (as CaCO 3 ) = N Alk eq. wt. CaCO3 1000 eq. wt. CaCO3 = 50 eq. wt. CaCO3 = 50 N b V b = N a V a N b V b = N a V a
9. The following equation can be used to express concentration as CaCO 3 : The following equation can be used to express concentration as CaCO 3 :
10. Purpose of the Experiment Purpose of the Experiment To become familiar with the concept of alkalinity and its measurement in water To become familiar with the concept of alkalinity and its measurement in water
11. Materials Materials Burette, 25 ml Burette, 25 ml Porcelain dish Porcelain dish Magnetic stirrer and rod Magnetic stirrer and rod Beaker, 150 ml Beaker, 150 ml Pipetter Pipetter Measuring cylinder, 100 ml Measuring cylinder, 100 ml pH meter pH meter 0.02N Sulphuric acid 0.02N Sulphuric acid Methyl Orange indicator Methyl Orange indicator Phenolphthalein indicator Phenolphthalein indicator Tap water sample Tap water sample Sweet water sample Sweet water sample Synthetic samples Synthetic samples
12. Procedure 1 - Indicator Method Procedure 1 - Indicator Method Pipette exactly 100 ml of sample into a porcelain dish and drop in a magnetic rod. Pipette exactly 100 ml of sample into a porcelain dish and drop in a magnetic rod. Mount a 25 ml burette and fill it to the mark with 0.02N sulphuric acid solution. Mount a 25 ml burette and fill it to the mark with 0.02N sulphuric acid solution. Add 5 drops of Phenolphthalein indicator to the sample. If the solution turns pink, add acid slowly till pink color disappears. Record the volume of acid in milliliters as P. Add 5 drops of Phenolphthalein indicator to the sample. If the solution turns pink, add acid slowly till pink color disappears. Record the volume of acid in milliliters as P. Add 5 drops of Methyl Orange indicator to the same sampling at the end of the first titration and add 0.02N sulphuric acid slowly till orange color turns to pink. Record this volume as M. Then, T = P+M. Add 5 drops of Methyl Orange indicator to the same sampling at the end of the first titration and add 0.02N sulphuric acid slowly till orange color turns to pink. Record this volume as M. Then, T = P+M.
13. Procedure 2 - Potentiometric Method (pH meter) Procedure 2 - Potentiometric Method (pH meter) Pipette exactly 100 ml of sample into a 150 ml beaker and drop in a magnetic rod. Pipette exactly 100 ml of sample into a 150 ml beaker and drop in a magnetic rod. Fill the burette with 0.02N sulfuric acid solution. Fill the burette with 0.02N sulfuric acid solution. If the pH of the sample is above 8.3 add 0.02N sulphuric acid slowly till pH 8.3. Record the volume of acid as P. If the pH of the sample is above 8.3 add 0.02N sulphuric acid slowly till pH 8.3. Record the volume of acid as P. Continue addition of acid till the pH of the sample reaches 4.5. Record volume of the acid as M. Then, T = P+M. Continue addition of acid till the pH of the sample reaches 4.5. Record volume of the acid as M. Then, T = P+M.
14. Calculation of Alkalinity Species Calculation of Alkalinity Species Condition OH CO 3 = HCO 3 1. P = T T 0 0 2. P = 1/2T 0 2P 0 3. P > 1/2T (2P-T) 2(T-P) 0 4. P < 1/2T 0 2P (T-2P) 5. P = 0 0 0 T