Discovering the Periodic Table

Dmitri Mendeleev determined the properties of every known element, arranged them in order of increasing atomic mass and discovered the periodic trends.
- Uploaded on | 0 Views
-
 leahcampbell
leahcampbell
About Discovering the Periodic Table
PowerPoint presentation about 'Discovering the Periodic Table'. This presentation describes the topic on Dmitri Mendeleev determined the properties of every known element, arranged them in order of increasing atomic mass and discovered the periodic trends.. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
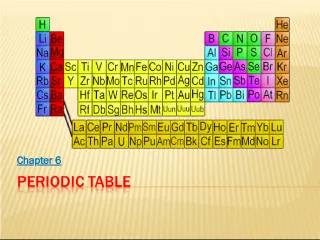
1. Trends in the Periodic Table
2. The Periodic Table Dmitri Mendeleev (1834-1907) determined the properties of every known element at the time Atomic Mass Density Colour Melting Point Boiling Point
3. The Periodic Table: Periods Mendeleev arranged the known elements in order of increasing atomic mass He found that the properties of the elements repeated at definite, or PERIODIC , intervals Na has similar properties to Li and K
4. The Modern Periodic Table: Atomic Number In 1915, the Periodic Table was reorganized based on the elements atomic structure Each element has an ATOMIC NUMBER , which is unique to each element The atomic number begins with H (1) in the upper-left hand corner, and moves from Left to Right
5. The Modern Periodic Table: Groups Vertical columns in the periodic table Chemical families Elements in a group share very similar properties Numbered from 1-18 Elements in the same GROUP have the same number of atomic SHELLS where VALENCE ELECTRONS (outer electrons) are held 1 2 3 4 3 2 1 0
6. Nonmetals Metals Metalloids Elements are grouped based on specific properties:
7. Metals Metals Non-Metals Non-Metals Metalloids Metalloids
8. The Modern Periodic Table: Characteristics of Groups The number of valence electrons in the elements is same in a group. The atomic radii increase from top to bottom Metallic Elements: Metallic character and chemical reactivity increase from top to bottom Non-Metallic Elements: Metallic character and chemical reactivity decrease from top to bottom
9. Elements are ordered in the periodic table according to reactivity and atomic number: Na and K react with Water Na K Mg and Ca React with Hydrochloric Acid (Same Group) Mg Ca F, Cl, I are gases F Cl I
10. The Modern Periodic Table: Groups of Elements 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 1 2
11. Group 1: Alkali Metals Very reactive metals that do not occur freely in nature (Cs, Fr the most reactive) Valence Electrons: One; ready to lose that one electron to bond with other elements. Properties: Malleable, ductile, good conductors of heat and electricity, softer than most metals Can explode if exposed to water.
12. Group 2: Alkaline Earth Metals Very reactive Valence Electrons: 2; can bond easily with other elements Not found free in nature
13. Groups 3-12: Transition Metals Properties: Ductile, malleable, conduct electricity and heat Valence Electrons: present in more than one shell Iron, cobalt, and nickel are the only elements known to produce a magnetic field.
14. Groups 13-15: Other Metals Properties: Ductile and malleable, solid, high density, opaque Valence Electrons : present in their outer shell.
15. Metalloids Along the stair-step line that distinguishes metals from non-metals Al is an exception as it is classified as an Other Metal Properties: Similar to metals and non-metals Si and Ge are semi-conductors (can carry an electrical charge under special conditions; used in calculators and computers)
16. Group 14-16: Non-Metals Properties: Do not conduct electricity or heat very well, brittle, not malleable or ductile, no luster, do not reflect light Exist as gases (N, O) or solids (C, S)
17. Group 17: Halogens Salt-former, compounds containing halogens are called salts Valence Electrons: 7; will bond easily with Alkali Metals. Exist as solids (I, At), liquid (Br), and gas (F, Cl)
18. Group 18: Noble Gases Valence Electrons: 0; prevents gases from readily forming compounds Very stable because they have the maximum electrons in their outer shell
19. Rare Earth Elements Lanthanide and Actinide series (Group 3 and Period 6-7) One element of the lanthanide series and most of the elements in the actinide series are trans- uranium (synthetic or man-made)
20. Mendeleevs Periodic Law If the elements are arranged according to their atomic mass, a pattern can be seen in which similar properties occur regularly