Calculations of Solution Concentration
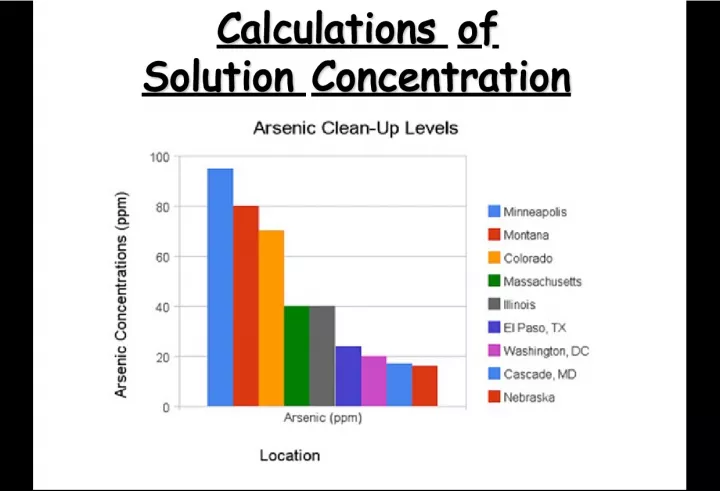

This article discusses various methods for calculating the concentration of a solution. It covers the use of CA standards, grams per liter, and molarity to determine the ratio of solute to solution volume
- Uploaded on | 8 Views
-
 topias
topias
About Calculations of Solution Concentration
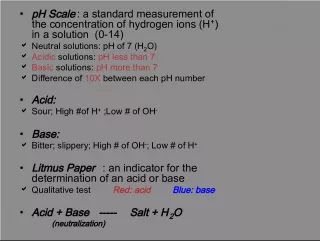
PowerPoint presentation about 'Calculations of Solution Concentration'. This presentation describes the topic on This article discusses various methods for calculating the concentration of a solution. It covers the use of CA standards, grams per liter, and molarity to determine the ratio of solute to solution volume. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
Slide1Calculations of Solution Concentration Calculations of Solution Concentration
Slide2CA Standards CA Standards
Slide3Calculations of Solution Concentration: Grams per Liter Calculations of Solution Concentration: Grams per Liter Grams per liter Grams per liter is the ratio of mass units of solute to volume (liters) of solution
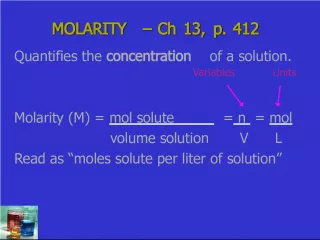
Slide4Calculations of Solution Concentration: Molarity Calculations of Solution Concentration: Molarity Molarity Molarity is the ratio of moles of solute to liters of solution
Slide5Calculations of Solution Concentration: Mass Percent Calculations of Solution Concentration: Mass Percent Mass percent Mass percent is the ratio of mass units of solute to mass units of solution, expressed as a percent
Slide6Calculations of Solution Concentration: Parts per Million Calculations of Solution Concentration: Parts per Million Parts per million Parts per million is the ratio of mass units of solute to mass units of solution, multiplied by one million (10 6 )
Slide7A Simplifying Assumption A Simplifying Assumption • 1 ml of water = 1 gram of water • 1 ml of water = 1 gram of water • 1000 ml of water = 1 liter = 1000 grams • 1000 ml of water = 1 liter = 1000 grams • Assume that solutions with water as the solvent have the density of pure water (1 mL = 1 gram) • Assume that solutions with water as the solvent have the density of pure water (1 mL = 1 gram) – It’s not true, but it’s close enough – It’s not true, but it’s close enough