Maintaining Adequate Blood Supply During Emergencies


The FDA seeks advice from the Blood Products Advisory Committee on increasing flexibility in Whole Blood collection during severe emergencies to ensure a safe and adequate blood supply.
- Uploaded on | 1 Views
-
 zoiicrist
zoiicrist
About Maintaining Adequate Blood Supply During Emergencies
PowerPoint presentation about 'Maintaining Adequate Blood Supply During Emergencies'. This presentation describes the topic on The FDA seeks advice from the Blood Products Advisory Committee on increasing flexibility in Whole Blood collection during severe emergencies to ensure a safe and adequate blood supply.. The key topics included in this slideshow are blood supply, emergency, transfusion, FDA, donation intervals,. Download this presentation absolutely free.
Presentation Transcript
1. Maintaining a Safe and Adequate Supply of Donated Blood During a Severe Emergency Alan E. Williams, Ph.D. Associate Director for Regulatory Affairs, OBRR, CBER, FDA Blood Products Advisory Committee August 3, 2011
2. Issue: FDA seeks the advice of the BPAC on current considerations to increase flexibility for the collection of Whole Blood for transfusion during a severe local, regional, or national emergency by permitting reduced interdonation intervals for eligible Whole Blood donors under specified conditions.
3. Session Outline Part I. Overview of government and private sector preparedness with respect to maintaining a safe and adequate supply of donated blood during emergencies
4. Session Outline Part II. FDA current considerations: FDA will introduce as current considerations two modifications to current Whole Blood inter-donation intervals for eligible donors when there is a potential or actual disruption of the blood supply in an emergency Scope of FDA considerations at this session does not specifically include: Emergency availability of platelets Medical aspects of fresh whole blood use in trauma Iron supplementation for donors Hemoglobin Standards and Maintaining Adequate Iron Stores in Blood Donors; Public Workshop November 8-9, 2011. Natcher Conference Center, NIH
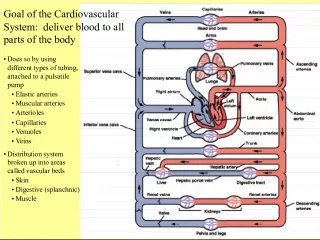
5. Key Characteristics of the US Blood Supply System u Unlike other developed nations, the US does not have a unified national blood system 93.8% collections by blood establishments Approximately 45% American Red Cross (Single FDA license, 36 Regions) Approximately 45% Americas Blood Centers (independent community blood centers) 6.2% Hospital-based collections
6. Key Characteristics of the US Blood Supply System u Most blood is stored at hospital transfusion services Hospital inventory levels (typically 5 - 7 days), reflect short-term availability Blood center inventory levels are flexible to support demand u Seasonal and sporadic drops in national inventory may occur under normal circumstances (e.g. RBC group O, platelets) u The major blood organizations have the ability to assess collector and customer inventories and provide supply coverage during shortages
7. Key Characteristics of the US Blood Supply - RBCs u Approximately 50% of transfusions in the US are necessary for non-elective patient support u The remaining 50% of RBC transfusions are elective (some uses are poorly characterized) u In 2008, 14,555,000 Whole Blood units were collected vs 1,926,000 Apheresis RBC units (NBCUS 2009)
8. Key Characteristics of the US Blood Supply - Platelets u 83.7% Platelets, Pheresis u Platelets prepared from 13.9% of Whole Blood units u Platelet transfusion increased 16.7% from 2007-09 u 5 day shelf life u Future emergency preparedness topic Source: NBCUS 2009
9. Key Characteristics of the US Blood Supply - DHHS u ESF #8 of National Response Plan (NRP) tasks HHS components to monitor and ensure the safety, availability and logistical requirements of blood, organs, and tissues. u HHS Advisory Committee for Blood Safety and Availability advises Secretary on safety and availability matters u HHS BASIS system receives summary reports from major blood collectors and a sample of large hospitals
10. Key Characteristics of the US Blood Supply - FDA u FDA has limited ability to influence blood production volume or distribution. Designs safety interventions so as to preserve critical supplies Maintains liaison to all AABB Disaster-Related Task Forces and Committees Receives daily BASIS reports during shortages upon request u FDA provides regulations, guidance and other measures to promote blood availability during shortages
11. Key Characteristics of the US Blood Supply Vulnerabilities u Severe blood shortages or dislocations of available blood supply, if they occur, could (very quickly) become a safety issue. u Disruptions could occur at several levels Unavailability of test facilities (US 9/11) Loss of transportation systems (US 9/11) Loss/Disruption of communications systems (US 9/11) Loss of electricity/long-term cold storage Unavailability of blood establishment staff due to logistical challenges or other obligations (New Zealand and Japan earthquake experiences) Donors/staff may be sheltered-in-place - e.g radiation, infection, violence ( Theoretical ) Disruption of IT systems ( Theoretical)
12. Key Characteristics of the US Blood Supply Existing Response Network u AABB Interorganizational Disaster Task Force Liaison relationship with HHS Designated in ESF-8 of NRP for blood supply logistics and the coordination of national public blood messages on the need to donate Level 1 members convene by telephone conference within one hour of an event (including representatives from affected blood establishments) Level 1 includes all major blood organizations, HHS, FDA, CDC, DOD The AABB Interorganizational Disaster Task Force has been activated multiple times (TOPOFF exercises, hurricanes, proactive planning for large events (political conventions, superbowl, inauguration, etc)
13. Key Characteristics of the US Blood Supply Existing Response Network u AABB Pandemic Influenza Task Force Proactive pandemic planning AABB Pandemic Influenza Task Force has proposed FDA regulatory flexibility during pandemic-related shortages (Wide range of regulatory flexibilities proposed to FDA) Task Force proposed reduction of interdonation intervals to meet blood supply needs during a pandemic
14. Regulatory Interventions to Preserve the Blood Availability Many internal and external discussions What? - Interventions When? - Triggers How? Mechanisms Existing Regulations and Guidances
15. Emergency Provisions for Blood Use in Medical Emergencies u 21 CFR 606.151(e) - Procedures to expedite transfusion in life-threatening emergencies. .. complete documentation justifying the emergency actionshall be signed by the physician (compatibility testing) u 21 CFR 606.160(b)(3)(v) Emergency release of blood, including signature of requesting physician . u 21 CFR 610.40(g) may be released or shipped prior to completion of testing in following circumstances .properly label the blood complete the tests as soon as possible and provide results to consignee: (1) Only in appropriately documented medical emergency situations :
16. 21 CFR 640.120 Alternative Procedures (Variances) The Director, Center for Biologics Evaluation and Research may approve an exception or alternative to any requirement in subchapter F of Chapter I regarding blood, blood components, or blood products Requested by individual manufacturer (21 CFR 601.12 prior approval supplement when licensed facility) List of approved variances available on FDA web site (e.g. alternate storage facility following hurricane)
17. Proposed modifications to 21 CFR 640.120(b) u 2007 FDA Proposed Rule Director of the Center for Biologics Evaluation and Research to issue an exception or alternative to the regulations in the event of a public health emergency. ( May apply to multiple blood establishments in specified locations) Only when a variance is necessary to assure the availability of blood, blood components, and blood products Current consideration is for FDA to issue an emergency measures guidance that would serve as a basis for blood establishment planning, but which would only activate in an emergency
18. November 2010 Guidance for Industry: u Training of Back-Up Personnel, Assessment of Blood Donor Suitability and Reporting Certain Changes to an Approved Application FDA recommends that blood establishments train personnel who may be called upon to assist in emergency response activities, including back-up personnel Training may include participation in emergency preparedness training and exercises and instruction on roles and responsibilities in a severe local or national emergency
19. Whole Blood Interdonation Interval Established in Regulation u In accordance with 21 CFR 640.3(b), Whole Blood donors are not allowed to donate more than one time in an eight week period; except that under 21 CFR 640.3(f), a donor may donate Whole Blood more than once in eight weeks if at the time of donation the person is examined and certified by a physician to be in good health
20. BPAC Proposal #1 Four week Whole Blood Interdonation Interval u For use under emergency conditions only. u Manual collection of a second Whole Blood (WB) unit no less than 4 weeks since the last WB collection, (i.e. instead of eight weeks) from donors who qualify based upon a conservative two unit RBC apheresis collection nomogram at the time of the second donation. u Physician review not required for 2nd donation. u Donation with a reduced interdonation interval limited to one time per year u Applicable only to donors who have not provided other collections (e.g. plateletpheresis, plasmapheresis) in the interval prior to the collection of the second WB unit. (Must also meet blood establishment SOP limits for RBC loss) u Subsequent deferral from all blood donations for sixteen weeks after the second WB donation.
21. BPAC Proposal #2 48 Hour Whole Blood Interdonation Interval u For use under emergency conditions only. u Manual collection of a second Whole Blood (WB) unit no less than 48 hours since the last WB donation from donors who qualify based upon a conservative two unit RBC apheresis collection nomogram at the time of the second donation. u Physician review recommended prior to 2nd donation u Donation with a reduced interdonation interval limited to one time per year . u Applicable only to donors who have not provided other collections (e.g. plateletpheresis, plasmapheresis) in the interval prior to the collection of the second WB unit. (Must also meet blood establishment SOP limits for RBC loss) u Subsequent deferral from all donations for sixteen weeks after the second WB donation
22. Rationale for Consideration of Reduced Interdonation Intervals for Whole Blood Collection u Double RBC apheresis is a rapidly growing portion of RBC supply and the procedures are safe and well-tolerated by donors u Use of a conservative RBC collection nomogram supports safe collection of Whole Blood units with a shortened interdonation interval in parallel with current FDA-cleared practices for double unit RBC apheresis u Two unit Whole Blood collection provides advantages of simplicity that may support blood collection operations during a severe emergency
23. Limitation of Red Cell (and Iron) Loss u Red Blood Cell loss from two 500 ml Whole Blood collections approximates a total of 420 mL, which can be removed safely through the use of a conservative nomogram Weight Height Hematocrit Hemoglobin Males 150 lbs. 5'1" 42 14.0 Females 175 lbs. 5'5' 42 14.0 u The nomogram helps to provide assurance that a smaller person would not be placed at risk for excessive RBC or fluid loss. u The nomogram-based eligibility criteria are more stringent for female donors due to the increased prevalence of low iron stores among female repeat donors.
24. Limitation of Fluid Volume Loss u A 500 ml Whole Blood collection results in 500ml volume loss, and total volume is replenished quickly by the body. u The minimum four week and 48 hour interdonation intervals under consideration for Whole Blood unit collection are viewed as sufficient for complete replacement of the intravascular fluid volume. u This assumption is backed by extensive experience with safety of plasmapheresis under current donation standards (2x/week with an interval of not less than 48 hours).
25. Blood Establishment Implementation Possibilities u Four week WB interdonation interval Records of eligible and willing donors tagged during normal operations (likely Group O) Pre-identified donors called if needed in an emergency u 48 hour WB interdonation interval Eligible and willing donors identified at WB donation during an emergency (likely Group O) Donors called back (or respond to appeal) if severe shortages exist.
26. Advantages offered by Whole Blood Collection in a Severe Emergency u Supports maintaining/increasing Group O WB collections during infrastructure disruptions Electricity Transportation Testing facilities u Supports continued collection operations despite absence of specialized staff u Pre-identifies potential willing repeat donors during sheltering in place
27. Advantages offered by Whole Blood Collection in a Severe Emergency u Simplicity in maintaining on-site WB collection supplies for emergency use Just in time inventory replenishment for most supplies now common, but subject to disruption u Close proximity of collection intervals for individual donors optimizes safety if incompletely tested units needed during appropriately documented medical emergency situations 21 CFR 610.40(g)
28. Session Outline A. Introduction and FDA Current Considerations, Alan Williams, Ph.D., OBRR, FDA B. History of Blood Utilization in Emergency Situations, John Hess, M.D., University of Maryland School of Medicine
29. Session Outline (cont.) C. Overview of Current Emergency Response Systems to Preserve Blood Availability Department of Health and Human Services Responsibilities under Emergency Support Function # 8, Jerry Holmberg, Ph.D., Senior Advisor for Blood, Office of the Assistant Secretary for Health, HHS Role of the AABB Interorganizational Task Force on Domestic Disasters and Acts of Terrorism, Jamie Blietz, AABB Practical Aspects of Emergency Response in Blood Establishments, Louis Katz, M.D. Mississippi Valley Regional Blood Center
30. Session Outline (cont.) Physiology/Donor Response to Reduced Interdonation Intervals, John Feiner, M.D., UCSF Blood Center Experience with Double Red Cell Collections by Apheresis, Peter Tomasulo, M.D. Blood Systems, Inc.
31. Questions for the Committee: Question 1. Do the available data support the safety of collecting two Whole Blood units during a severe emergency with an interdonation interval of >=4 weeks from donors who are eligible under the proposed nomogram and under the conditions outlined below including: Limited to established, repeat donors Physician review not required for second donation Sixteen week deferral from all donations following second collection No other donations between the two Whole Blood collections Complies with RBC donation limits established in blood establishment SOPs
32. Questions for the Committee: u Question 2. Do the available data support the safety of collecting two Whole Blood units during a severe emergency with an interdonation interval of >= 48 hours from donors who are eligible under the proposed nomogram (applies to first donation) and have a hemoglobin greater than or equal to 12.5 at the second donation including the conditions outlined below: Limited to established, repeat donors Sixteen week deferral from all donations following second collection. No other donations between the two Whole Blood collections Complies with RBC donation limits established in blood establishment SOPs
33. Questions for the Committee: u Question 3. u Should a p hysician review be required for the second donation?