Molarity

Molarity is a unit of measurement that quantifies the concentration of a solution. It is represented by the symbol M and is defined as the number of moles of solute (n) per liter of solution (
- Uploaded on | 1 Views
-
 tammy
tammy
About Molarity
PowerPoint presentation about 'Molarity'. This presentation describes the topic on Molarity is a unit of measurement that quantifies the concentration of a solution. It is represented by the symbol M and is defined as the number of moles of solute (n) per liter of solution (. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
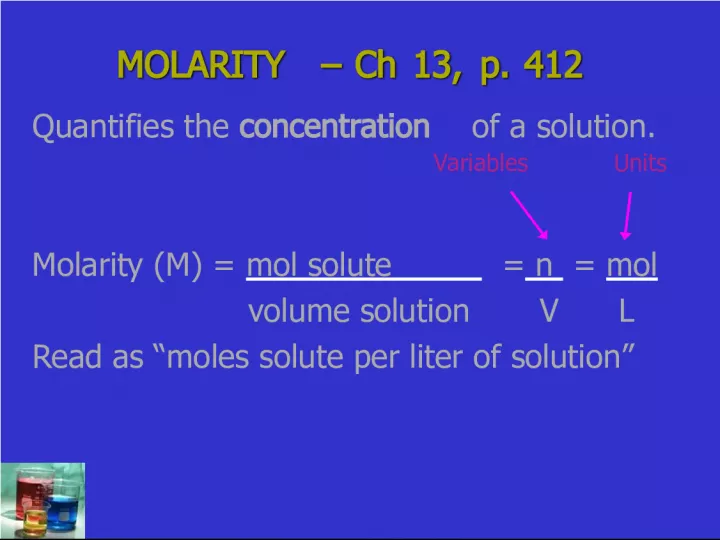
Slide1MOLARITY – Ch 13, p. 412 MOLARITY – Ch 13, p. 412 Quantifies the concentration of a solution. Molarity (M) = mol solute = n = mol volume solution V L Read as “moles solute per liter of solution” Units Variables
Slide2MOLARITY – Ch 13 MOLARITY – Ch 13 Example 1: A bottle labeled “0.2 M NaCl” reads as “0.2 molar sodium chloride solution.”
Slide3MOLARITY – Ch 13 MOLARITY – Ch 13 Example 1: A bottle labeled “0.2 M NaCl” reads as “0.2 molar sodium chloride solution.” This means 1 L of the solution contains 0.2 moles NaCl.
Slide4MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Calculate the molarity of a solution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution.
Slide5Example 2: Calculate the molarity of asolution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution. Given:
Slide6Example 2: Calculate the molarity of asolution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution. Given: mass = 5.844 g
Slide7Example 2: Calculate the molarity of asolution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution. Given: mass = 5.844 g V = 200.0 mL
Slide8Example 2: Calculate the molarity of asolution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution. Given: mass = 5.844 g V = 200.0 mL Unknown:
Slide9Example 2: Calculate the molarity of asolution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution. Given: mass = 5.844 g V = 200.0 mL Unknown: M
Slide10Example 2: Calculate the molarity of asolution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution. Given: mass = 5.844 g V = 200.0 mL Unknown: M Need:
Slide11MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Calculate the molarity of a solution prepared by dissolving 5.844 g NaCl in enough water to make a 200.0 mL solution. Given: mass = 5.844 g V = 200.0 mL Unknown: M Need: molar mass NaCl
Slide12MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Given: mass = 5.844 g V = 200.0 mL = 0.2000 L Unknown: M Need: molar mass NaCl 58.443 g/mol M = n = 5.844 g V
Slide13MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Given: mass = 5.844 g V = 200.0 mL = 0.2000 L Unknown: M Need: molar mass NaCl M = n = 5.844 g mol V 58.443 g
Slide14MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Given: mass = 5.844 g V = 200.0 mL = 0.2000 L Unknown: M Need: molar mass NaCl M = n = 5.844 g mol V 58.443 g
Slide15MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Given: mass = 5.844 g V = 200.0 mL = 0.2000 L Unknown: M Need: molar mass NaCl M = n = 5.844 g mol V 0.2000 L 58.443 g
Slide16MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Given: mass = 5.844 g V = 200.0 mL = 0.2000 L Unknown: M Need: molar mass NaCl M = n = 5.844 g mol 0.49997 M V 0.2000 L 58.443 g
Slide17MOLARITY – Ch 13 MOLARITY – Ch 13 Example 2: Given: mass = 5.844 g V = 200.0 mL = 0.2000 L Unknown: M Need: molar mass NaCl M = n = 5.844 g mol 0.5000 M V 0.2000 L 58.443 g
Slide18MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH.
Slide19MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given:
Slide20MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given: V = 5000.0 mL
Slide21MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given: V = 5000.0 mL molarity = 0.250 M
Slide22MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given: V = 5000.0 mL molarity = 0.250 M Unknown: n, mol NaOH
Slide23MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given: V = 5000.0 mL molarity = 0.250 M Unknown: n, mol NaOH Need:
Slide24MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given: V = 5000.0 mL molarity = 0.250 M Unknown: n, mol NaOH Need: M = n V
Slide25MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given: V = 5000.0 mL molarity = 0.250 M Unknown: n, mol NaOH Need: M = n V
Slide26MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Calculate the moles of NaOH present in 5000.0 mL of 0.250 M NaOH. Given: V = 5000.0 mL molarity = 0.250 M Unknown: n, mol NaOH Need: M = n n = M • V V
Slide27MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Given: V = 5000.0 mL molarity = 0.250 M Unknown: n, mol NaOH Need: M = n n = M • V V 0.250 M
Slide28MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Given: V = 5000.0 mL molarity = 0.250 M Unknown: n, mol NaOH Need: M = n n = M • V V 0.250 mol L
Slide29MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Given: V = 5000.0 mL = 5.0000 L molarity = 0.250 M Unknown: n, mol NaOH Need: M = n n = M • V V 0.250 mol L
Slide30MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Given: V = 5000.0 mL = 5.0000 L molarity = 0.250 M Unknown: n, mol NaOH Need: M = n n = M • V V 0.250 mol 5.0000 L L
Slide31MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Given: V = 5000.0 mL = 5.0000 L molarity = 0.250 M Unknown: n, mol NaOH Need: M = n n = M • V V 0.250 mol 5.0000 L L
Slide32MOLARITY – Ch 13 MOLARITY – Ch 13 Example 3: Given: V = 5000.0 mL = 5.0000 L molarity = 0.250 M Unknown: n, mol NaOH Need: M = n n = M • V V 0.250 mol 5.0000 L 1.25 mol L
Slide33MOLARITY – Ch 13 MOLARITY – Ch 13 Example 4: Calculate the volume of 0.50 M HCl that would contain 2.0 mol HCl. YOU TRY IT!
Slide34MOLARITY – Ch 13 MOLARITY – Ch 13 Example 4: Given: n = 2.0 mol HCl molarity = 0.50 M Unknown: V Need: M = n V = n V M 2.0 mol L 4.0 L 0.50 mol
Slide35MOLARITY – Ch 13 MOLARITY – Ch 13 Example 5: How would you prepare 100 mL of 0.50 M HCl from concentrated 12 M HCl? YOU TRY IT!
Slide36MOLARITY – Ch 13 MOLARITY – Ch 13 Example 5: How would you prepare 100 mL of 0.50 M HCl from concentrated 12 M HCl? Use C 1 V 1 = C 2 V 2
Slide37MOLARITY – Ch 13 MOLARITY – Ch 13 Example 5: How would you prepare 100 mL of 0.50 M HCl from concentrated 12 M HCl? Use C 1 V 1 = C 2 V 2 Given: C 1 = 0.50 M V 1 = 100mL C 2 = 12 M
Slide38MOLARITY – Ch 13 MOLARITY – Ch 13 Example 5: How would you prepare 100 mL of 0.50 M HCl from concentrated 12 M HCl? Use C 1 V 1 = C 2 V 2 Given: C 1 = 0.50 M V 1 = 100mL C 2 = 12 Unknown:
Slide39MOLARITY – Ch 13 MOLARITY – Ch 13 Example 5: How would you prepare 100 mL of 0.50 M HCl from concentrated 12 M HCl? Use C 1 V 1 = C 2 V 2 Given: C 1 = 0.50 M V 1 = 100mL C 2 = 12 Unknown: V 2 =
Slide40MOLARITY – Ch 13 MOLARITY – Ch 13 Example 5: How would you prepare 100 mL of 0.50 M HCl from concentrated 12 M HCl? Use C 1 V 1 = C 2 V 2 C 1 V 1 = V 2 C 2 Given: C 1 = 0.50 M V 1 = 100mL C 2 = 12 Unknown: V 2 =