Understanding Electron Distribution in Molecules through Lewis Electron Dot Structures

In order to determine how atoms bond covalently in molecules, it is important to understand electron distribution. This is typically depicted through Lewis electron dot structures, which show how
- Uploaded on | 2 Views
-
 elisha
elisha
About Understanding Electron Distribution in Molecules through Lewis Electron Dot Structures
PowerPoint presentation about 'Understanding Electron Distribution in Molecules through Lewis Electron Dot Structures'. This presentation describes the topic on In order to determine how atoms bond covalently in molecules, it is important to understand electron distribution. This is typically depicted through Lewis electron dot structures, which show how. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
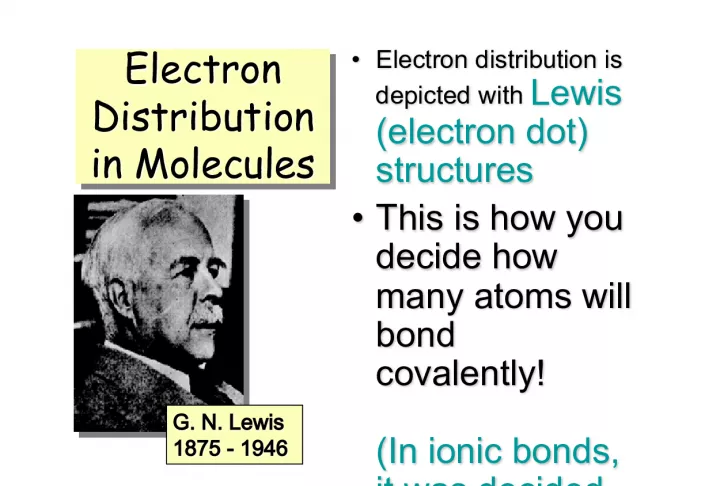
Slide1ElectronDistribution in Molecules Electron Distribution in Molecules Electron Distribution in Molecules Electron Distribution in Molecules • Electron distribution is depicted with Lewis (electron dot) structures • Electron distribution is depicted with Lewis (electron dot) structures • This is how you decide how many atoms will bond covalently! (In ionic bonds, it was decided with charges) • This is how you decide how many atoms will bond covalently! (In ionic bonds, it was decided with charges) G. N. Lewis G. N. Lewis 1875 - 1946 1875 - 1946
Slide2Bond and Lone Pairs Bond and Lone Pairs Bond and Lone Pairs Bond and Lone Pairs • Valence electrons are distributed as shared or BOND PAIRS and unshared or LONE PAIRS. • Valence electrons are distributed as shared or BOND PAIRS and unshared or LONE PAIRS. • •• • •• H Cl lone pair (LP) shared or bond pair This is called a LEWIS structure. This is called a LEWIS structure.
Slide3Bond Formation Bond Formation Bond Formation Bond Formation A bond can result from an overlap of atomic orbitals on neighboring atoms. A bond can result from an overlap of atomic orbitals on neighboring atoms. Cl H H Cl •• • • •• •• • • •• + Overlap of H (1s) and Cl (2p) Note that each atom has a single, unpaired electron. Note that each atom has a single, unpaired electron.
Slide4Review of Valence Electrons Review of Valence Electrons Review of Valence Electrons Review of Valence Electrons • Remember from the electron chapter that valence electrons are the electrons in the OUTERMOST energy level … that ’ s why we did all those electron configurations! • Remember from the electron chapter that valence electrons are the electrons in the OUTERMOST energy level … that ’ s why we did all those electron configurations! • B is 1s 2 2s 2 2p 1 ; so the outer energy level is 2, and there are 2+1 = 3 electrons in level 2. These are the valence electrons! • B is 1s 2 2s 2 2p 1 ; so the outer energy level is 2, and there are 2+1 = 3 electrons in level 2. These are the valence electrons! • Br is [Ar] 4s 2 3d 10 4p 5 How many valence electrons are present? • Br is [Ar] 4s 2 3d 10 4p 5 How many valence electrons are present?
Slide5Review of Valence Electrons Review of Valence Electrons Review of Valence Electrons Review of Valence Electrons Number of valence electrons of a main (A) group atom = Group number Number of valence electrons of a main (A) group atom = Group number
Slide6Steps for Building a Dot Structure Steps for Building a Dot Structure Steps for Building a Dot Structure Steps for Building a Dot Structure Ammonia, NH 3 Ammonia, NH 3 1. Decide on the central atom; never H. Why? 1. Decide on the central atom; never H. Why? If there is a choice, the central atom is atom of lowest affinity for electrons. (Most of the time, this is the least electronegative atom … in advanced chemistry we use a thing called formal charge to determine the central atom. But that ’ s another story!) Therefore, N is central on this one If there is a choice, the central atom is atom of lowest affinity for electrons. (Most of the time, this is the least electronegative atom … in advanced chemistry we use a thing called formal charge to determine the central atom. But that ’ s another story!) Therefore, N is central on this one 2. Add up the number of valence electrons that can be used. 2. Add up the number of valence electrons that can be used. H = 1 and N = 5 H = 1 and N = 5 Total = (3 x 1) + 5 Total = (3 x 1) + 5 = 8 electrons / 4 pairs = 8 electrons / 4 pairs
Slide73.Form a single bond between the central atom and each surrounding atom (each bond takes 2 electrons!) 3. Form a single bond between the central atom and each surrounding atom (each bond takes 2 electrons!) H H H N Building a Dot Structure Building a Dot Structure H •• H H N 4. Remaining electrons form LONE PAIRS to complete the octet as needed (or duet in the case of H). 4. Remaining electrons form LONE PAIRS to complete the octet as needed (or duet in the case of H). 3 BOND PAIRS and 1 LONE PAIR. 3 BOND PAIRS and 1 LONE PAIR. Note that N has a share in 4 pairs (8 electrons), while H shares 1 pair. Note that N has a share in 4 pairs (8 electrons), while H shares 1 pair.
Slide85.Check to make sure there are 8 electrons around each atom except H. H should only have 2 electrons. This includes SHARED pairs. 5. Check to make sure there are 8 electrons around each atom except H. H should only have 2 electrons. This includes SHARED pairs. Building a Dot Structure Building a Dot Structure 6. Also, check the number of electrons in your drawing with the number of electrons from step 2. If you have more electrons in the drawing than in step 2, you must make double or triple bonds. If you have less electrons in the drawing than in step 2, you made a mistake! 6. Also, check the number of electrons in your drawing with the number of electrons from step 2. If you have more electrons in the drawing than in step 2, you must make double or triple bonds. If you have less electrons in the drawing than in step 2, you made a mistake! H •• H H N
Slide9Carbon Dioxide, CO 2 Carbon Dioxide, CO 2 Carbon Dioxide, CO 2 Carbon Dioxide, CO 2 1. Central atom = 1. Central atom = 2. Valence electrons = 2. Valence electrons = 3. Form bonds. 3. Form bonds. 4. Place lone pairs on outer atoms. 4. Place lone pairs on outer atoms. This leaves 12 electrons (6 pair). This leaves 12 electrons (6 pair). 5. Check to see that all atoms have 8 electrons around it except for H, which can have 2. 5. Check to see that all atoms have 8 electrons around it except for H, which can have 2. C 4 e- O 6 e- X 2 O’s = 12 e- Total: 16 valence electrons C 4 e- O 6 e- X 2 O’s = 12 e- Total: 16 valence electrons
Slide10Carbon Dioxide, CO 2 Carbon Dioxide, CO 2 Carbon Dioxide, CO 2 Carbon Dioxide, CO 2 6. There are too many electrons in our drawing. We must form DOUBLE BONDS between C and O. Instead of sharing only 1 pair, a double bond shares 2 pairs. So one pair is taken away from each atom and replaced with another bond. 6. There are too many electrons in our drawing. We must form DOUBLE BONDS between C and O. Instead of sharing only 1 pair, a double bond shares 2 pairs. So one pair is taken away from each atom and replaced with another bond. C 4 e- O 6 e- X 2 O’s = 12 e- Total: 16 valence electrons C 4 e- O 6 e- X 2 O’s = 12 e- Total: 16 valence electrons How many are in the drawing? How many are in the drawing?