Mole Calculations and Visualizing a Chemical Reaction of Na and Cl2


This presentation covers mole calculations and the visualization of a chemical reaction between sodium (Na) and chlorine gas (Cl2) to form sodium chloride (Na
- Uploaded on | 2 Views
-
 florian
florian
About Mole Calculations and Visualizing a Chemical Reaction of Na and Cl2
PowerPoint presentation about 'Mole Calculations and Visualizing a Chemical Reaction of Na and Cl2'. This presentation describes the topic on This presentation covers mole calculations and the visualization of a chemical reaction between sodium (Na) and chlorine gas (Cl2) to form sodium chloride (Na. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
Slide1Mole Calculations
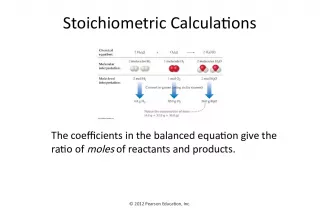
Slide2?Visualizing a Chemical Reaction Na + Cl 2 NaCl ___ mole Cl 2 ___ mole NaCl ___ mole Na 2 10 5 10 2 10 5 10
Slide3___ mole NaCl10 Visualizing a Chemical Reaction Na + Cl 2 NaCl ___ mole Cl 2 ___ mole Na 2 10 5 2
Slide4Formation of Ammonia
Slide5Proportional RelationshipsI have 5 eggs. How many cookies can I make? 3/4 c. brown sugar 1 tsp vanilla extract 2 eggs 2 c. chocolate chips Makes 5 dozen cookies. 2 1/4 c. flour 1 tsp. baking soda 1 tsp. salt 1 c. butter 3/4 c. sugar 5 eggs 5 doz. 2 eggs = 12.5 dozen cookies Ratio of eggs to cookies Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem 150 cookies
Slide6Proportional Relationships• Stoichiometry • Stoichiometry – mass relationships between substances in a chemical reaction – based on the mole ratio • Mole Ratio • Mole Ratio – indicated by coefficients in a balanced equation 2 Mg + O 2 2 MgO 2 Mg + O 2 2 MgO Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Slide7Stoichiometry Steps1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. – Mole ratio - moles moles – Molar mass - moles grams – Molarity - moles liters soln – Molar volume - moles liters gas Core step in all stoichiometry problems!! – Mole ratio - moles moles 4. Check answer. Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Slide81 mol of a gas=22.4 Lat STP Molar Volume at STP S tandard T emperature & P ressure 0°C and 1 atm Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Slide9Molar Volume at STPMolar Mass ( g/mol ) 6.02 10 23 particles/mol MASS IN GRAMS MOLES NUMBER OF PARTICLES LITERS OF SOLUTION Molar Volume ( 22.4 L/mol) LITERS OF GAS AT STP Molarity (mol/L) Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Slide10Stoichiometry ProblemsHow many moles of KClO 3 must decompose in order to produce 9 moles of oxygen gas? 9 mol O 2 2 mol KClO 3 3 mol O 2 = 6 mol KClO 3 2KClO 3 2KCl + 3O 2 ? mol 9 mol Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Slide11How many moles of KClO3 must decompose in order to produce 9 moles of oxygen gas? 9 mol O 2 2 mol KClO 3 3 mol O 2 = 6 mol KClO 3 2KClO 3 2KCl + 3O 2 ? mol 9 mol Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem O 2 KClO 3 x m o l K C l O 3 = 9 m o l O 2 = 6 mol KClO 3 2 mol KClO 3 3 mol O 2 6 mol
Slide12How many grams of KClO3 are required to produce 9.00 L of O 2 at STP? 9.00 L O 2 1 mol O 2 22.4 L O 2 = 32.8 g KClO 3 2 mol KClO 3 3 mol O 2 122.55 g KClO 3 1 mol KClO 3 ? g 9.00 L Stoichiometry Problems 2KClO 3 2KCl + 3O 2 Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Slide13How many grams of KClO3 are required to produce 9.00 L of O 2 at STP? ? g 9.00 L Stoichiometry Problems 2KClO 3 2KCl + 3O 2 Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem O 2 KClO 3 x g K C l O 3 = 9 . 0 0 L O 2 22.4 L O 2 = 32.8 g KClO 3 1 mol O 2 2 mol KClO 3 3 mol O 2 122.55 g KClO 3 1 mol KClO 3 32.8 g
Slide14How many grams of KClO3 are required to produce 9.00 L of O 2 at STP? 9.00 L O 2 1 mol O 2 22.4 L O 2 = 32.8 g KClO 3 2 mol KClO 3 3 mol O 2 122.55 g KClO 3 1 mol KClO 3 ? g 9.00 L 2KClO 3 2KCl + 3O 2 Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem O 2 KClO 3 x g K C l O 3 = 9 . 0 0 L O 2 22.4 L O 2 = 32.8 g KClO 3 1 mol O 2 2 mol KClO 3 3 mol O 2 122.55 g KClO 3 1 mol KClO 3 32.8 g
Slide15How many grams of silver will be formed from12.0 g copper? 12.0 g Cu 1 mol Cu 63.55 g Cu = 40.7 g Ag Cu + 2 AgNO 3 2 Ag + Cu(NO 3 ) 2 2 mol Ag 1 mol Cu 107.87 g Ag 1 mol Ag 12.0 g ? g Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem Cu Ag x g A g = 1 2 . 0 g C u 63.55 g Cu = 40.7 g Ag 1 mol Cu 2 mol Ag 1 mol Cu 107.87 g Ag 1 mol Ag 40.7 g
Slide16useAvogadro’s number use molar mass Mole Calculations Volume, cm 3 Mass, g Moles Atoms use density g cm 3 x mol g x atoms mol x A graduated cylinder holds 25.4 cm 3 of mercury. If the density of mercury at 25 o C is 13.534 g / cm 3 , how many moles of mercury are in the cylinder? HINT: Volume of solids/liquids and moles are not directly connected. You must first use the density to convert the volume to a mass, and then derive the quantity of mercury, in moles, from the mass. Finally, the number of atoms is obtained from the number of moles. Kotz & Treichel, Chemistry & Chemical Reactivity , 3 rd Edition , 1996, page 93 H o w m a n y a t o m s o f m e r c u r y a r e t h e r e ?
Slide17Th e r e f o r e , t h e m a s s o f m e r c u r y i s f o u n d t o b e e q u i v a l e n t t o 3 4 4 g o f m e r c u r y . Volume, cm 3 Mass, g Moles Atoms use density use molar mass use Avogadro’s number g cm 3 x mol g x . 13.534 g Hg 1 cm 3 Hg 25.4 cm 3 Hg = 344 g Hg K n o w i n g t h e m a s s , y o u c a n n o w f i n d t h e q u a n t i t y i n m o l e s . . 1 mol Hg . 200.6 g Hg 344 g Hg = 1.71 mol Hg F i n a l l y , b e c a u s e y o u k n o w t h e r e l a t i o n b e t w e e n a t o m s a n d m o l e s ( A v o g o d r o ’ s n u m b e r ) , y o u c a n n o w f i n d t h e n u m b e r o f a t o m s i n t h e s a m p l e . . 6.02 x 10 23 atoms Hg . 1 mol Hg 1.71 mol Hg = 1.03 x 10 24 atoms Hg A A B C B C Kotz & Treichel, Chemistry & Chemical Reactivity , 3 rd Edition , 1996, page 93 A graduated cylinder holds 25.4 cm 3 of mercury. If the density of mercury at 25 o C is 13.534 g / cm 3 , how many moles of mercury are in the cylinder? How many atoms of mercury are there? 344 g Hg 1.71 mol Hg atoms mol x
Slide182 Na + Cl2 2 NaCl 2 grams 1 gram 2 grams W R O N G Violates Law of Conservation of Matter 2 atoms 1 molecule 2 molecules* * B e t t e r n a m e w o u l d b e “ f o r m u l a u n i t ” 2 moles 1 mole 2 moles 100 g x L x g Na Cl 2 x L C l 2 = 1 0 0 g N a 23 g Na = 49 L Cl 2 1 mol Na 1 mol Cl 2 2 mol Na 22.4 L Cl 2 1 mol Cl 2 48.69 L R i g h t s i d e o f r o o m … c a l c u l a t e h o w m a n y g r a m s o f N a C l w i l l b e p r o d u c e d f r o m 1 0 0 g o f N a . L e f t s i d e o f r o o m … c a l c u l a t e h o w m a n y g r a m s o f N a C l w i l l b e p r o d u c e d f r o m 4 8 . 6 9 L o f C l 2 . Na NaCl x g N a C l = 1 0 0 g N a 23 g Na = 254 g NaCl 1 mol Na 2 mol NaCl 2 mol Na 58.5 g NaCl 1 mol NaCl Cl 2 NaCl x g N a C l = 4 8 . 6 9 L C l 2 22.4 L Cl 2 = 254 g NaCl 1 mol Cl 2 2 mol NaCl 1 mol Cl 2 58.5 g NaCl 1 mol NaCl
Slide19StoichiometryKClO 3 KCl + O 2 3 2 2 500 g x g x L KClO 3 O 2 x L O 2 = 5 0 0 g K C l O 3 122.5 g KClO 3 = 137 L O 2 1 mol KClO 3 3 mol O 2 2 mol KClO 3 22.4 L O 2 1 mol O 2 KClO 3 KCl x g K C l = 5 0 0 g K C l O 3 122.5 g KClO 3 = 304 g KCl 1 mol KClO 3 2 mol KCl 2 mol KClO 3 74.5 g KCl 1 mol KCl (304 g) (196 g) x g O 2 = 1 3 7 L O 2 22.4 L O 2 = 196 g O 2 1 mol O 2 32 g O 2 1 mol O 2 137 L
Slide20Stoichiometry2 TiO 2 + 4 Cl 2 + 3 C CO 2 + 2 CO + 2 TiCl 4 4.55 mol x mol C Cl 2 x m o l C = 4 . 5 5 m o l C l 2 4 mol Cl 2 = 6.07 mol Cl 2 3 mol C C TiO 2 x m o l e c u l e s T i C l 4 = 1 1 5 g T i O 2 80 g TiO 2 = 8.66 x 10 23 molecules TiCl 4 1 mol TiO 2 2 mol TiCl 4 2 mol TiO 2 6.02 x 10 23 molecules TiCl 4 1 mol TiCl 4 x g T i O 2 = 4 . 5 5 m o l C 3 mol C = 243 g TiO 2 1 mol TiO 2 80 g TiO 2 2 mol TiO 2 How many moles of chlorine will react with 4.55 moles of carbon? H o w m a n y g r a m s o f t i t a n i u m ( I V ) o x i d e w i l l r e a c t w i t h 4 . 5 5 m o l e s o f c a r b o n ? x g H o w m a n y m o l e c u l e s o f T i C l 4 w i l l r e a c t w i t h 1 1 5 g T i O 2 ? TiO 2 TiCl 4 x molecules 115 g
Slide21Stoichiometry Problems 1Keys Keys Stoichiometry Problems 1 Stoichiometry Problems 1
Slide226.02x 10 23 atoms H 2 O 6.02x10 23 molecules H 2 O Which has more atoms: 30 g aluminum metal or 18 mL distilled water? x a t o m s A l = 3 0 g A l 27 g Al = 6.69 x 10 23 atoms Al 1 mol Al 6.02 x 10 23 atoms Al 1 mol Al H o w m a n y a t o m s o f a l u m i n u m a r e i n 3 0 g o f a l u m i n u m ? Al x a t o m s A l = 3 0 g A l 27 g Al = 6.69 x 10 23 atoms Al 6.02 x 10 23 atoms Al Al x a t o m s = 1 8 m L H 2 O 1000 mL H 2 O = 1.45 x 10 21 atoms 1 L H 2 O 1 mol H 2 O 22.4 L H 2 O H o w m a n y a t o m s a r e i n 1 8 m L o f w a t e r ? H 2 O x a t o m s = 1 8 m L H 2 O 1 mL H 2 O = 1.81 x 10 24 atoms 1 g H 2 O 1 mol H 2 O 18 g H 2 O H o w m a n y a t o m s a r e i n 1 8 m L o f w a t e r ? 1 mol H 2 O 3 atoms 1 molecule H 2 O 1 moL H 2 O 6.02x10 23 molecules H 2 O 3 atoms 1 molecule H 2 O W R O N G W R O N G Recall, density of water LITERS is ONLY used for GASES @ STP LITERS is ONLY used for GASES @ STP