Understanding the Organization and Phases of Matter

This article explores the different ways in which matter is organized and the various phases it can exist in, including solids, liquids, gases, and plasmas. It also covers the properties of pure substances, compounds, and elements, as well as the subatomic particles that make them up.
- Uploaded on | 1 Views
-
 khloebrakus
khloebrakus
About Understanding the Organization and Phases of Matter
PowerPoint presentation about 'Understanding the Organization and Phases of Matter'. This presentation describes the topic on This article explores the different ways in which matter is organized and the various phases it can exist in, including solids, liquids, gases, and plasmas. It also covers the properties of pure substances, compounds, and elements, as well as the subatomic particles that make them up.. The key topics included in this slideshow are Matter, Mixtures, Homogeneous Solutions, Heterogeneous Mixtures, Pure Substances, Elements, Compounds, Atoms, Nucleus, Electrons, Protons, Neutrons, Quarks, Phase Differences, Solids, Liquids, Gases, Plasma,. Download this presentation absolutely free.
Presentation Transcript
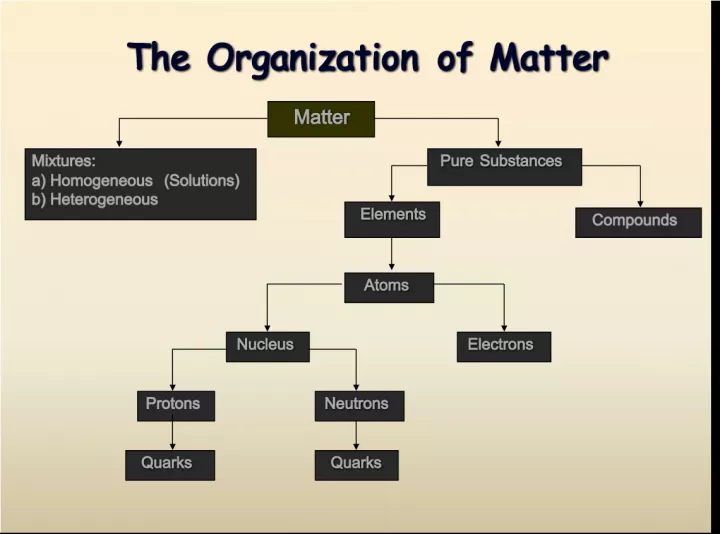
1. The Organization of Matter The Organization of Matter Matter Matter Mixtures: a) Homogeneous (Solutions) b) Heterogeneous Pure Substances Pure Substances Compounds Elements Elements Atoms Atoms Nucleus Nucleus Electrons Electrons Protons Neutrons Neutrons Quarks Quarks Quarks Quarks
2. Phase Differences Phase Differences Solid Solid definite volume and shape; particles packed in fixed positions. Liquid Liquid definite volume but indefinite shape; particles close together but not in fixed positions Gas Gas neither definite volume nor definite shape; particles are at great distances from one another Plasma Plasma high temperature, ionized phase of matter as found on the sun.
3. Properties of Matter Properties of Matter Volume Mass Energy Content (think Calories!) Extensive properties Extensive properties depend on the amount of matter that is present. Intensive properties Intensive properties do not depend on the amount of matter present. Melting point Boiling point Density
4. Separation of a Mixture Separation of a Mixture The constituents of the mixture retain their identity and may be separated by physical means. The constituents of the mixture retain their identity and may be separated by physical means.
5. Separation of a Mixture Separation of a Mixture The components of dyes such as ink may be separated by paper chromatography. The components of dyes such as ink may be separated by paper chromatography.
6. Separation of a Mixture by Distillation Separation of a Mixture by Distillation
7. Separation of a Compound Separation of a Compound The Electrolysis of water Water Hydrogen + Oxygen 2 H 2 O 2 H 2 + O 2 Reactant Products chemical Compounds must be separated by chemical means. With the application of electricity, water can be separated into its elements