Understanding Matter and Elements


This unit, Unit D, focuses on elements and their combinations as well as providing a clear understanding of what matter is. It covers topics such as the definition of matter, the characteristics of matter, and the
- Uploaded on | 4 Views
-
 cosima
cosima
About Understanding Matter and Elements
PowerPoint presentation about 'Understanding Matter and Elements'. This presentation describes the topic on This unit, Unit D, focuses on elements and their combinations as well as providing a clear understanding of what matter is. It covers topics such as the definition of matter, the characteristics of matter, and the. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
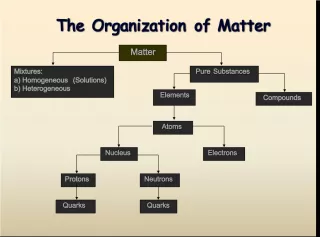
Slide1Unit D Unit D Elements and their Combinations Elements and their Combinations
Slide2What is matter? What is matter? a. Something that has mass and takes up space a. Something that has mass and takes up space b. Something that is important b. Something that is important c. Something that is a problem c. Something that is a problem d. Something that takes up no space and is invisible d. Something that takes up no space and is invisible
Slide3What is matter made up of? What is matter made up of? a. Organs a. Organs b. Cells b. Cells c. Atoms c. Atoms d. Links d. Links
Slide4Which element is a metal? Which element is a metal? a. Oxygen a. Oxygen b. Gold b. Gold c. Carbon c. Carbon d. Helium d. Helium
Slide5Which is NOT a property of a metal? Which is NOT a property of a metal? a. Shiny a. Shiny b. Can bend b. Can bend c. Melts easily c. Melts easily d. Gets hot d. Gets hot
Slide6What is the smallest number of parts in a compound? What is the smallest number of parts in a compound? a. 1 a. 1 b. 2 b. 2 c. 3 c. 3 d. 4 d. 4
Slide7You can see an atom with You can see an atom with a. Your eye a. Your eye b. A special telescope b. A special telescope c. A magnifying glass c. A magnifying glass d. A special microscope d. A special microscope
Slide8Atoms may combine to form a(n) Atoms may combine to form a(n) a. Mixture a. Mixture b. Molecule b. Molecule c. Nucleus c. Nucleus d. Element d. Element
Slide9What does periodic mean What does periodic mean a. Happening sometimes a. Happening sometimes b. Happening all the time b. Happening all the time c. Happening at regular times c. Happening at regular times d. Happening at the end d. Happening at the end
Slide10What element is a building block of matter in living things? What element is a building block of matter in living things? a. Carbon a. Carbon b. Hydrogen b. Hydrogen c. Helium c. Helium d. Oxygen d. Oxygen
Slide11Which one of the following is NOT one of your senses? Which one of the following is NOT one of your senses? a. Hearing a. Hearing b. Sight b. Sight c. Touch c. Touch d. Thinking d. Thinking
Slide12Which group lists the physical properties of materials? Which group lists the physical properties of materials? a. Shape, color, ability to float a. Shape, color, ability to float b. Smell, density, ability to rust b. Smell, density, ability to rust c. Color, texture, ability to burn c. Color, texture, ability to burn d. Taste, texture ability to rust d. Taste, texture ability to rust
Slide13Which is an example of a chemical change? Which is an example of a chemical change? a. Cutting paper a. Cutting paper b. Making ice b. Making ice c. Burning wood c. Burning wood d. Boiling water d. Boiling water
Slide14A compound is made up of A compound is made up of a. An element a. An element b. Different kinds of atoms b. Different kinds of atoms c. The same kind of atom c. The same kind of atom d. Salt and water d. Salt and water
Slide15Water is a Water is a a. Compound a. Compound b. An element b. An element c. A mixture c. A mixture d. A solution d. A solution
Slide16What will happen in this beaker? What will happen in this beaker? a. Nothing a. Nothing b. The water will boil b. The water will boil c. The water will disappear c. The water will disappear d. The sugar will dissolve d. The sugar will dissolve
Slide17The temperature at which a solid changes to a liquid is called the The temperature at which a solid changes to a liquid is called the a. Boiling point a. Boiling point b. Celsius scale b. Celsius scale c. Fahrenheit scale c. Fahrenheit scale d. Melting point d. Melting point
Slide18Some foods taste because they contain a type of chemical called an acid. Which food contains acid? Some foods taste because they contain a type of chemical called an acid. Which food contains acid? a. Ice cream a. Ice cream b. Lemons b. Lemons c. Peas c. Peas d. Chicken d. Chicken
Slide19What is NOT a property of salt? What is NOT a property of salt? a. It is white a. It is white b. It is made of tiny crystals b. It is made of tiny crystals c. It tastes sour c. It tastes sour d. It mixes in water d. It mixes in water
Slide20The amount of space an object takes up is called its The amount of space an object takes up is called its a. Temperature a. Temperature b. Mass b. Mass c. Volume c. Volume d. Shape d. Shape
Slide21Which kind of substance has no definite shape and probably a large amount of space between its particles? Which kind of substance has no definite shape and probably a large amount of space between its particles? a. Solid a. Solid b. Liquid b. Liquid c. Gas c. Gas d. Water d. Water
Slide22Which substance has no definite shape but has a definite volume? Which substance has no definite shape but has a definite volume? a. Iron fillings a. Iron fillings b. Liquid water b. Liquid water c. Nitrogen gas c. Nitrogen gas d. Salt crystals d. Salt crystals
Slide23The process of liquid water changing to a gas is called The process of liquid water changing to a gas is called a. Freezing a. Freezing b. Precipitation b. Precipitation c. Melting c. Melting d. Vaporization d. Vaporization
Slide24Which is a property of glass? Which is a property of glass? a. It is clear a. It is clear b. It can bend b. It can bend c. It is soft c. It is soft d. It is a liquid at room temperature d. It is a liquid at room temperature
Slide25Chocolate milk is Chocolate milk is a. A chemical a. A chemical b. A compound b. A compound c. A mixture c. A mixture d. A reaction d. A reaction
Slide26Hydrogen combines with oxygen to make water. A scientist calls the hydrogen and oxygen Hydrogen combines with oxygen to make water. A scientist calls the hydrogen and oxygen a. Ingredients a. Ingredients b. Reactants b. Reactants c. Products c. Products d. Energy d. Energy
Slide27A difference between a physical change and a chemical change is that in a chemical change A difference between a physical change and a chemical change is that in a chemical change a. A new substance forms a. A new substance forms b. No new substance forms b. No new substance forms c. Only the shape of the substance changes c. Only the shape of the substance changes d. Only the size of the substance changes d. Only the size of the substance changes
Slide28What do solids, liquids, and gases have in common? What do solids, liquids, and gases have in common? a. They can be squeezed to take up less space a. They can be squeezed to take up less space b. People can see them b. People can see them c. They have shape c. They have shape d. They are made up of particles d. They are made up of particles