Zeroth Law of Thermodynamics: A Universal Law for Measurement


This article explains the zeroth law of thermodynamics proposed by P M V Subbarao, a professor of mechanical engineering at IIT Delhi. The law lays the foundation for measuring temperature and understanding thermal equilibrium. The article emphasizes the importance of the law and its significance in the study of thermodynamics.
- Uploaded on | 10 Views
-
 pilar
pilar
About Zeroth Law of Thermodynamics: A Universal Law for Measurement
PowerPoint presentation about 'Zeroth Law of Thermodynamics: A Universal Law for Measurement'. This presentation describes the topic on This article explains the zeroth law of thermodynamics proposed by P M V Subbarao, a professor of mechanical engineering at IIT Delhi. The law lays the foundation for measuring temperature and understanding thermal equilibrium. The article emphasizes the importance of the law and its significance in the study of thermodynamics.. The key topics included in this slideshow are Zeroth law, thermodynamics, measurement, temperature, thermal equilibrium,. Download this presentation absolutely free.
Presentation Transcript
1. Zeroth Law of Themodynamics P M V Subbarao Professor Mechanical Engineering Department I I T Delhi An Universal Law for Measurement
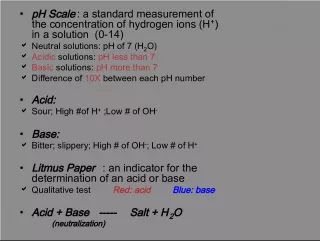
2. Zeroth Law of Thermodynamics Every system in this universe spontaneously move towards equilibrium with surroundings. If A and C are in thermal equilibrium with B, then A is in thermal equilibrium with C. Maxwell [1872] Practically this means that all three are at the same temperature. A basis for comparison of effect of temperatures.
3. Demonstration of Zeroth Law When Two bodies have equality of temperature with a third body, they in turn have equality of temperature with each other. BRASS Copper If the substance that composes the system is in thermal equilibrium, the temperature will be the same throughout the entire system, and we may speak of the temperature as a property of the system
4. Construction of A Thermometer
5. Thermometers with Electrical Output Liquid in Glass Thermometer Thermocouple Thermometer
6. How Long it takes to achive Zeroth Law? Conservation of Energy during a time dt Heat in = Change in energy of thermocouple
7. Define Time constant Sudden Immersion of Thermometer in a Known Constant Temperature System
10. Response of A Thermo-couple in A Constant Temperature System
11. Response of A Thermo-couple in A Ramp Temperature System T s (t)=Ct
12. Format of Examinations Minor Examinations: each of one hour duration. Major Examination: Two hours duration. All the examinations are open hand written notes examinations. All kinds of your own hand written notes are allowed to be used during examinations. (Class notes, Tutorial notes, etc.) No printed or photocopied material is allowed. Printed copies of my lectures are also not allowed. Hand outs supplied by me are allowed.
13. Weightage of examinations A Grand total of 200 points will be considered. Following distribution is sued for individual evaluations: Minor-1 30 Minor 2 40 Major 70 Tutorial notes evaluation 30 Assignments 30
14. Extension of Zeroth Law
15. Mechanical Equilibrium When Two bodies have equality of pressure with a third body, they in turn have equality of pressure with each other. LPG Cylinder Oxygen Cylinder
16. Mechanical Equilibrium of A Finite size System If a system is in mechanical equilibrium, there is no changing tendency for the pressure at any point. There will be a variation in pressure with elevation because of the influence of gravitational forces, although under equilibrium conditions there will be no tendency for the pressure at any location to change. In many thermodynamic problems, this variation in pressure with elevation is so small that is can be neglected.
17. Chemical Equilibrium A system is in chemical equilibrium when there is no tendency for the quantities of species to change. When Two bodies have equality of concentration with a third body, they in turn have equality of concentration with each other.
18. Chemical Sensors Can we selectively detect chemicals? Can we also detect classes of chemicals? An electronic tongue or nose! Chemical Sensors covers a wide category of devices used to monitor, measure, test, analy s e concentration.
19. Control Methods for Forest Fire
20. Thermodynamic Equilibrium When a system is in equilibrium regarding all possible changes of state, we say that the system is in Thermodynamic Equilibrium . The properties of a system can be measured, when system is at equilibrium. Definition of a system using certain observable, macroscopic properties is known as description of The state. Some familiar ones are temperature, pressure, and density. How many properties are required to completely describe the state of a system?
21. State Postulate The state postulate for a simple, pure substance states that the equilibrium state can be determined by specifying any two independent intensive properties.
22. Change of State Whenever one or more of the properties of a system change, we say that a change in state has occurred.
23. Change of State Whenever one or more of the properties of a system change, we say that a change in state has occurred. Change of state due to an infinitesimal deviations from thermodynamic equilibrium can be precisely identified.
24. Economics of Change of State Every change of state of a system consumes resource (Energy). Detail record of a change of state is essential to efficiently use a given resource. Keeping a record of change of state of a system is called as detailing the process in thermodynamics.
25. Economics of Utilization of Energy Why So? The paths followed are different
26. Thermodynamic Process The path of succession of states through which the system passes is called the process. Quasi-equilibrium process is a collection of changes, in which the deviation from thermodynamic equilibrium is infinitesimal. All the states the system passes through during a quasi-equilibrium process may be considered equilibrium states.
27. Process Executed by A Control Volume