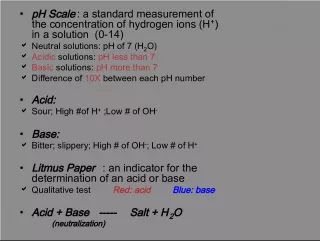
Understanding the Concept of pH and the Self-Ionization of Water
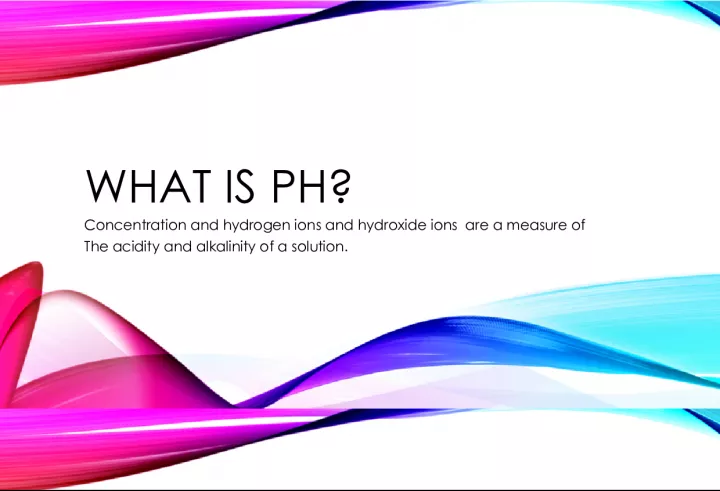

pH concentration, as well as the concentration of hydrogen ions (H+) and hydroxide ions (OH-), are crucial measures of the acidity and alkalinity
- Uploaded on | 1 Views
-
 jamie
jamie
About Understanding the Concept of pH and the Self-Ionization of Water
PowerPoint presentation about 'Understanding the Concept of pH and the Self-Ionization of Water'. This presentation describes the topic on pH concentration, as well as the concentration of hydrogen ions (H+) and hydroxide ions (OH-), are crucial measures of the acidity and alkalinity. The key topics included in this slideshow are . Download this presentation absolutely free.
Presentation Transcript
Slide1WHAT IS PH?Concentration and hydrogen ions and hydroxide ions are a measure of The acidity and alkalinity of a solution.
Slide2HOW WAS THE CONCEPT OF PHDEVELOPED? H 2 O is a molecule. Therefore, pure water does not conduct electricity.
Slide3OR DOES IT?When pure water was found to conduct very small currents of electricity, the self-ionization of water was discovered.
Slide4SELF-IONIZATION OF WATERH 2 O H + + OH - Water can dissociate into Hydronium ions and hydroxide ions.
Slide5HOW MUCH DISSOCIATION ISTHERE? [H + ] = 1 x 10 -7 M [OH - ] = 1 x 10 -7 M Therefore: [H + ] x [OH - ] = 1 x 10 -14 M
Slide6[H+ ]X [OH - ] = 1 X 10 -14 M Is called the Ion-product constant AKA: k w
Slide7ION PRODUCT CONSTANTK w = 1 x 10 -14 M
Slide8ACIDITY IS A MEASURE OF THE [H+ ] OF A SOLUTION. If the [H + ] of a solution is 1.0 x 10 -5 M, is the solution acidic, basic or neutral? 1.0x 10 -5 M is greater than 1.0 x 10 -7 M Therefore the solution is acidic
Slide9PRACTICE: ACIDIC, BASIC ORNEUTRAL? 1. [H + ] = 6.0 x 10 -10 M 2. [H + ] = 2.0 x 10 -7 M 3. [OH - ] = 1.0 x 10 -7 M
Slide10SOLVE THESE PROBLEMS USING K W = [H + ] X [OH - ] AND DETERMINE WHETHER SOLUTION IS ACIDIC, NEUTRAL OR BASIC 1. [OH - ] = 1 x 10 -3 2. [OH - ] = 2.0 x 10 -11 3. [OH - ] = 3.5 x 10 -7
Slide11THE PH SCALERanges from 0 -14. 0 – 6.9 = acidic 7.0 = neutral 7.1 – 14.0 = basic (alkaline)
Slide12SO WHERE DO THESE NUMBERSCOME FROM? pH = - log [H + ] That’s where they come from!
Slide13WHAT IS THE PH?1. [H + ] = 1.0 x 10 - 4 M 2. [H + ] = 1.0 x 10 -2 M 3. [H + ] = 1.0 x 10 -10 M 4. [H + ] = 1.0 x 10 -7 M 5. [H + ] = 1.0 x 10 -14 M
Slide14ANSWERS1. 4 2. 2 3. 10 4. 7 5. 14
Slide15GET YOUR CALCULATORS OUTAND PRACTICE. 1. [H + ] = 0.0045 M 2. [H + ] = 8.7 x 10 -6 M 3. [H + ] = 0.0015M 4. [H + ] = 1.2 x 10 -3 M
Slide16ANSWERS1. PH = 2.35 2. PH = 5.06 3. PH = 2.82 4. PH = 2.92
Slide17FIND THE PH AND DETERMINE IF ITIS ACID BASIC OR NEUTRAL • [H + ] = 1.5 x 10 -9 • [H + ] = 3.4 x 10 -2 • [H + ] = 5.5 x 10 -11 • [H + ] = 1.0 x 10 -7
Slide18YOU CAN ALSO CALCULATE PHFROM [OH - ] If you are given [OH - ] use [H + ] = 1 x 10 -14 [OH - ] To find hydrogen ion concentration then solve for pH pH = –log [H + ]
Slide19LET’S PRACTICECalculate the pH of a solution with a [OH - ] of 3.5 x 10 -9
Slide20EXIT SLIP: YOUR NAME HERE……1. Find the pH of a solution with an hydroxide concentration of [OH - ] = 2.9 x 10 -5 2. A solution has a hydrogen ion concentration of [H + ] = 5.0 x 10 -12 . Is it acidic, basic or neutral?
Slide21BRAINPOP